Abstract
ABT-888, a PARP-inhibitor in clinical trials, potentiates DNA-damaging agents. We developed and validated, according to FDA guidelines, an LC-MS assay for sensitive, accurate and precise quantitation of ABT-888 and its metabolite M8 in 0.2 ml human plasma. After ethyl acetate extraction, separation is achieved with a hydro-Synergi column and a 0.1% formic acid in acetonitrile/water-gradient. Detection uses electrospray, positive-mode ionization mass spectrometry. Between 10 (LOQ) and 1,000 ng/mL, accuracy was 95.5–98.5% for ABT-888 and 91.4–100.9% for M8, and precision was 0.1–4.9% for ABT-888 and 0–13.7% for M8. The assay is being applied to samples generated in several clinical trials.
Keywords: ABT-888, Mass Spectrometry, PARP-inhibitor
1 Introduction
Poly-(ADP-ribosyl)ation is involved in many cellular processes, including: differentiation; gene regulation; protein degradation; replication; transcription; and overall maintenance of genomic stability [1]. A family of 18 Poly(ADP-ribose) polymerases (PARPs) has been identified, but only the most abundant, PARP-1 and PARP-2, which are both nuclear enzymes, are activated by DNA damage [1]. PARP-1 and PARP-2 play a critical role in the DNA damage response process by regulating a variety of DNA repair mechanisms. Elevated levels of PARP in cancer cells compared to normal cells have been linked to drug resistance and the overall ability of cancer cells to survive genotoxic stress [2].
Inhibition of PARP sensitizes tumor cells to cytotoxic agents that cause DNA damage that would normally be repaired by the base excision repair system. These cytotoxics include: alkylating agents, such as temozolomide and cyclophosphamide; platinum analogues, such as cisplatin, carboplatin and oxaliplatin; and topoisomerase I poisons, such as irinotecan and topotecan [3–6]. Furthermore, PARP inhibition sensitizes cancer cells to radiation [2,3,7]. Consequently, PARP inhibition may improve the efficacy of DNA-damaging cytotoxic therapies [2].
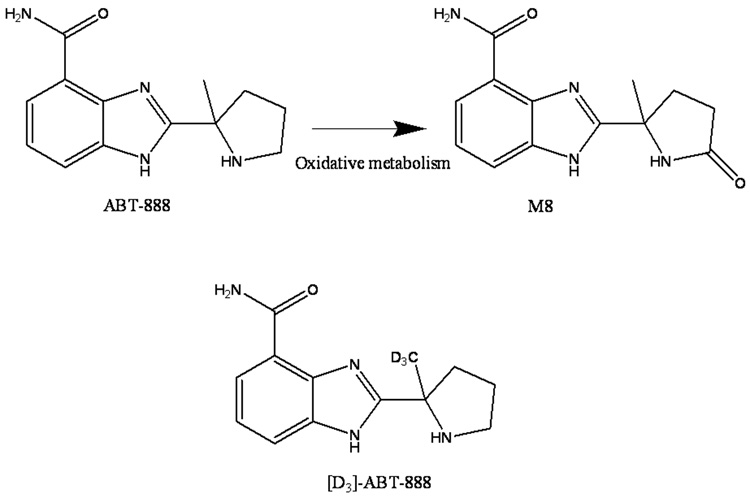
ABT-888 is a novel, orally active poly (ADP-ribose) polymerase (PARP) inhibitor, currently in clinical trials [6]. ABT-888 has an oral bioavailability of 56% to 92 % in mice, rats, dogs, and monkeys [3] and is excreted primarily via the urine, with approximately 50% as intact parent compound and another 15% as the inactive lactone metabolite M8 (Fig. 1) [4].
Fig. 1.
Structures of ABT-888 and its oxidative metabolite M8, and the structure of the internal standard [D3]-ABT-888. M8 may be formed from ABT-888 by the action of CYP1A1, 1A2, 2C9 and 2C19.
In a phase 0 clinical study, ABT-888 doses of 10–25 mg were associated with approximately 85% inhibition of PARP in tumor tissues and peripheral blood mononuclear cells [6].
As ABT-888 is undergoing more extensive clinical development, there is a need to evaluate its pharmacokinetics. To facilitate such an evaluation, we developed a simple, rapid and sensitive LC-MS assay for the quantitation of ABT-888 and M8 in human plasma and validated it according to the most recent FDA guidelines for bioanalytical method validation [8].
2 Experimental
2.1 Chemicals and reagents
ABT-888 (A-861695) (D0/D3 >99.8%), the internal standard ([D3]-ABT-888, D3/D0 >99.9%) and A925088 (the M8 metabolite) were graciously provided by Abbott Laboratories (Abbott Park, IL, USA). Acetonitrile (HPLC grade) and ethyl acetate (HPLC grade) were purchased from Fisher Scientific (Fairlawn, NJ, USA). Water was purified using a Q-gard® 1 Gradient Milli-Q system (18.2 MΩ.cm, Millipore, Billerica, MA, USA). Formic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA). Control human plasma was produced by centrifuging whole blood (Central Blood Bank, Pittsburgh, PA, USA) for 20 min at 2000 × g at room temperature. Nitrogen for evaporation of samples was purchased from Valley National Gases, Inc. (Pittsburgh, PA, USA). Nitrogen for mass spectrometrical applications was purified with a Parker Balston Nitrogen Generator (Parker Balston, Haverhill, MA, USA)
2.2 Chromatography
The LC system consisted of an Agilent (Palo Alto, CA, USA) 1100 autosampler and binary pump, a Phenomenex (Torrance, CA, USA) hydro Synergi (4 µm, 100 × 2 mm) column kept at ambient temperature, and a gradient mobile phase. Mobile phase solvent A was 0.1% (v/v) formic acid in acetonitrile, and mobile phase solvent B was 0.1 % (v/v) formic acid in water. The initial mobile phase composition of 2% solvent A and 98% solvent B was linearly increased to 30% solvent B from 0 to 10 min at a flow rate of 0.2 ml/min. Between 10 and 11 min, the percentage of solvent A was increased to 80%, while the flow rate was increased to 0.3 mL/min. Between 11 and 14 min, these conditions were mainatained (wash step). Between 14 and 15 min, the solvent composition was returned to 2% solvent A and 98% solvent B at a flow rate of 0.3 ml/min, followed by a re-equilibration period at these conditions until 25 min. Total run time was 25 min.
2.3 Mass spectrometry
Mass spectrometric detection was carried out using a ThermoFinnigan (San Jose, CA, USA) MSQ mass spectrometer with electrospray ionization in positive-ion mode. The settings of the mass spectrometer were as follows: capillary voltage 4.0 kV; cone voltage 10 V; and probe temperature 400 °C. In single ion monitoring mode, the m/z values monitored were 244.9, 247.9, and 258.9 for ABT-888, [D3]-ABT-888, and M8, respectively. The LC system and mass spectrometer were controlled by ThermoFinnigan Excalibur software (version 1.4), and data were collected with the same software. The analyte-to-internal standard ratio (response) was calculated for each standard by dividing the area of the analyte peak by the area of the internal standard peak.
2.4 Preparation of calibration standards and quality control samples
Stock solutions of ABT-888 and metabolite M8 were prepared at 1 mg/ml in acetonitrile:water (50:50, v/v) and stored at 4 °C. On assay days, this solution was serially diluted (in steps of 10-fold) with acetonitrile:water (50:50, v/v) to obtain the lower calibration working solutions. These calibration working solutions were diluted in human plasma to produce the following ABT-888 and M8 concentrations: 10, 30, 100, 300, 500, 750, and 1000 ng/ml (no precipitation of the analytes was observed). For each calibration series, zero and blank samples were also prepared from 200 µl of control plasma.
Quality control (QC) stock solutions were prepared independently from separate weightings of ABT-888 and M8 and stored at 4 °C. These solutions were diluted in human plasma to produce the following QC samples: QC low (QCL) 20 ng/ml; QC mid (QCM) 200 ng/ml; and QC high (QCH) 800 ng/ml.
2.5 Sample preparation
Ten µl of 10 µg/ml [D3]-ABT-888 (internal standard) in acetonitrile:water (50:50 v/v) and 1 ml of ethyl acetate were added sequentially to each tube of 200 µl standard, QC, or sample plasma. Samples were vortexed for 1 min on a Vortex Genie-2 set at 8 (Model G-560 Scientific Industries, Bohemia, NY, USA) and then centrifuged at 12,000 × g at room temperature for 5 min. The resulting supernatants were transferred to 12 mm × 75 mm borosilicate glass tubes and evaporated to dryness under a stream of nitrogen at 37 °C. Dried residues were re-dissolved in 100 µl of water. The solutions were sonicated for 2 min and transferred to autosampler vials, followed by injection of 5 µl into the LC-MS system.
2.6 Validation procedures
2.6.1 Calibration curve and lower limit of quantitation (LLQ)
Decreasing concentrations of ABT-888 and M8 were injected into the analytical system to determine the minimal concentration with a signal-to-noise ratio of at least 5:1. Calibration standards and blanks were prepared (see paragraph 2.4) and analyzed in triplicate to establish the calibration range with acceptable accuracy and precision. The analyte-to-internal standard ratio (response) was calculated for each sample by dividing the area of the analyte peak by the area of the internal standard peak. Standard curves of ABT-888 and M8 were constructed by plotting the analyte-to-internal standard ratio versus the known concentration of ABT-888 and M8, respectively, in each sample. Standard curves were fit by linear regression with weighting by 1/y2, followed by back-calculation of concentrations. The deviations of these back-calculated concentrations from the nominal concentrations, expressed as percentage of the nominal concentration, reflected the assay performance over the concentration range.
2.6.2 Accuracy and precision
The accuracy and precision of the assay were determined by analyzing samples with ABT-888 and M8 at the LLQ, QCL, QCM, and QCH concentrations in 8 replicates each in 3 analytical runs, together with an independently prepared, triplicate calibration curve. Accuracy was calculated at each test concentration as:
(mean measured concentration / nominal concentration) × 100%.
Assay precision was calculated by ANOVA as described[9] through SPSS 15.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Back-calculated concentrations of calibration and QC samples were entered with the run number as factor. From the resulting mean squares of the within runs and mean squares of the between runs, the intra-assay and inter-assay precisions were calculated.
2.6.3 Selectivity and specificity
To investigate whether endogenous matrix constituents interfered with the assay, six individual batches of control, drug-free human plasma were processed and analyzed according to the described procedures. Responses of ABT-888 and M8 at the LLQ concentrations were compared with the response of the blank samples.
2.6.4 Extraction recovery and ion-suppression
We determined the extraction recoveries of ABT-888 and M8 from plasma by comparing the absolute response of an extract of control plasma to which these analytes had been added after extraction with the absolute response of an extract of plasma to which the same amounts had been added before extraction. The ion-suppression of ABT-888 and M8 by plasma matrix components was defined as the decrease in signal when comparing the absolute response of an extract of control plasma to which ABT-888 and M8 had been added after the extraction with the absolute response of reconstitution solvent to which the same amount of each respective analyte had been added. Experiments were performed at the three QC concentrations, in triplicate.
2.6.5 Stability
Long-term stability experiments were performed in plasma at −80 °C for 3 months and in stock solution at 4 °C for 3 months. Stability in the stock solution was expressed as the percentage recovery of the stored solution relative to the fresh solution. The stabilities of ABT-888 and M8 in plasma at −80 °C were determined by assaying samples before and after 3 months of storage. In addition, the stabilities of ABT-888 and M8 in stock solution at room temperature for 4 h were determined in triplicate. All stability testing in plasma was performed in triplicate at the QCL, QCM and QCH concentrations. The effect of 3 freeze/thaw cycles on ABT-888 and M8 concentrations in plasma was evaluated by assaying samples after they had been frozen (−80 °C) and thawed on 3 separate days and comparing the results with those of freshly prepared samples. The stabilities of ABT-888 and M8 in plasma during sample preparation were evaluated by assaying samples before and after 4 h of storage at room temperature. To evaluate the stabilities of ABT-888 and M8 in reconstituted samples in the autosampler, we re-injected QC samples and calibration curves approximately 72 h after the first injection and compared the concentrations derived from the second injection with those derived from the first injection. Results of the second runs were expressed as a percentage of their respective values in the first runs.
2.6.6 Parallelism
To demonstrate parallelism, the ability to dilute samples from above the upper limit of quantitation to within the validated concentration range, plasma samples containing ABT-888 and M8 above the upper limit of quantitation were diluted to within the assay range. Plasma samples (N=3) with ABT-888 and M8 concentrations of 5 µg/mL were diluted 20-fold with control plasma and assayed.
2.7 Application of the assay
To show the applicability of the method, we used it to quantitate ABT-888 and its metabolite M8 in the plasma of a 64-year old male with lung cancer, who was treated with a 10 mg of ABT-888 p.o. in a phase I trial. Written informed consent, as approved by the University of Pittsburgh Institutional Review Board, was obtained before the patient participated. Heparinized blood was collected before ABT-888 administration and at 30, 60, and 90 min, and 2, 3, 4, 6, and 8 h thereafter. Blood was centrifuged at 1000 × g at 4 °C for 10 min. The resulting plasma was stored at −70 °C until analysis.
3 Results and Discussion
3.1 Validation of the assay
3.1.1 Chromatography
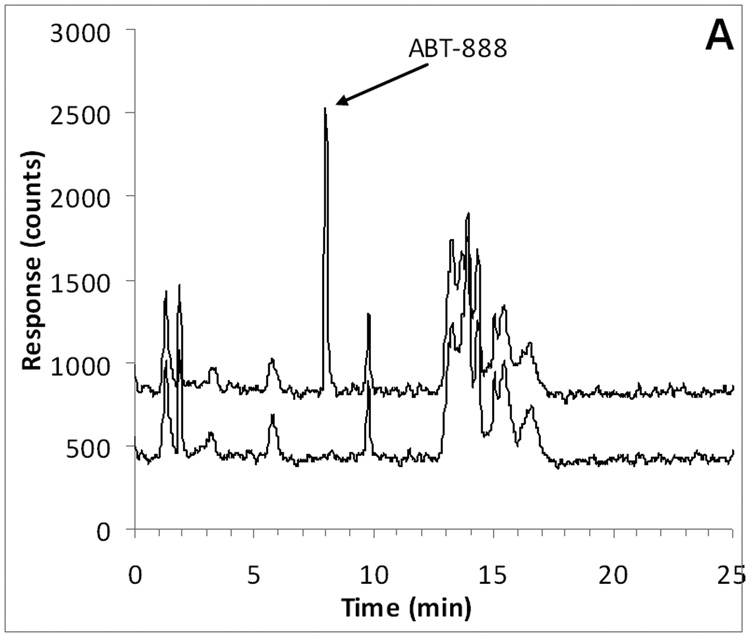
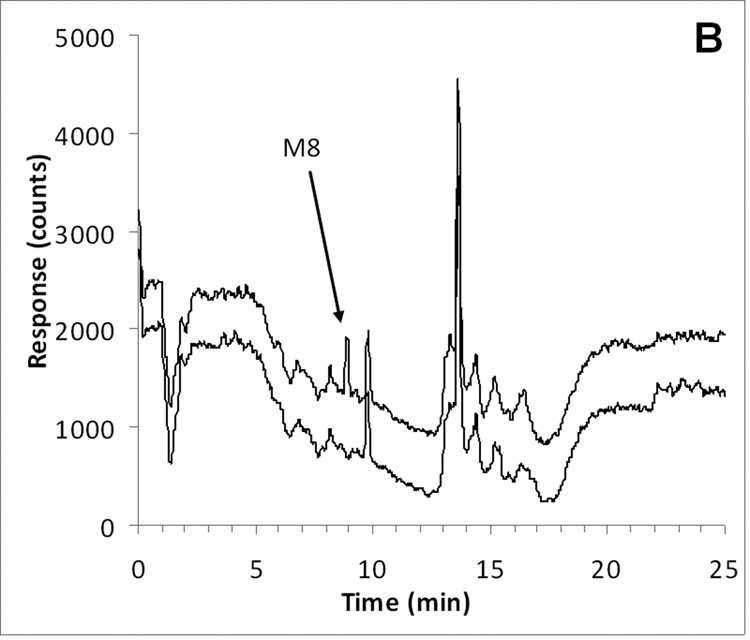
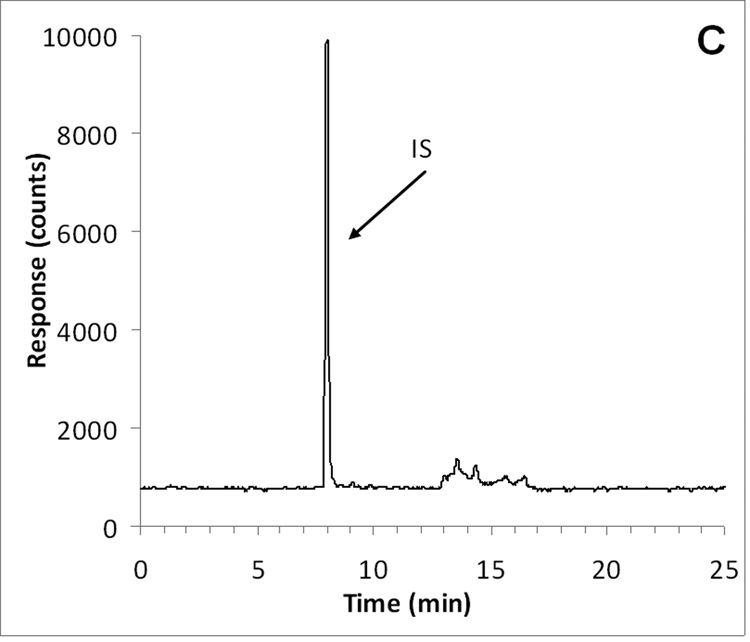
ABT-888 and the internal standard [D3]-ABT-888 had an identical retention time of approximately 8.0 min, corresponding to a capacity factor of 7.0 (with a void time of 1 min). M8 eluted slightly later at a retention time of 8.9 min, corresponding to a capacity factor of 7.9. Representative chromatograms of ABT-888, M8 (at the LLQ), and internal standard in plasma are displayed in Fig. 2.
Fig. 2.
Representative chromatograms of A) ABT-888 (m/z 245.2; 8.0 min) added to control plasma at the LLQ concentration of 10 ng/mL (top trace) and control human plasma (bottom trace with an offset of −400 counts); B) M8 (m/z 259.2; 8.9 min) added to control plasma at the LLQ concentration of 10 ng/mL (top trace) and control human plasma (bottom trace with an offset of −600 counts); C) [D3]-ABT-888 internal standard (m/z 248.2; 8.0 min) added to control plasma at the a concentration of 500 ng/mL.
3.1.2 Calibration curve and LLQ
According to the FDA guidelines for bioanalytical method validation [8], the calibration curve describes the concentration versus response relationship adequately if the observed deviation and precision are ≤20% for the LLQ and ≤15% for all other calibration concentrations. At least 4 out of 6 calibration points should meet the above criteria [8].
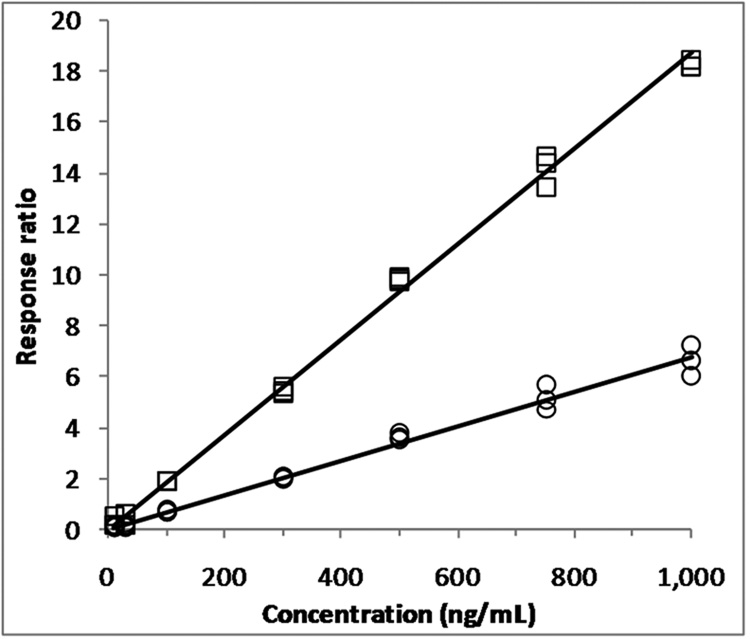
The selected assay range of 10–1000 ng/mL fulfilled the FDA criteria for the LLQ concentration and the calibration curve. Accuracies and precisions at the different calibration concentrations were determined from triplicate calibration curves on 3 separate days and are reported in Table 1. At most concentrations, the mean square of the within runs was greater than the mean square of the between runs, indicating that there was no significant additional variability due to the performance of the assay in different runs[9]. A representative calibration curve and corresponding correlation and regression coefficient are shown in Fig. 3.
Table 1.
Assay performance data of the calibration samples for ABT-888 and M8 in human plasma.
| Analyte | Concentration (ng/mL) | Accuracy (%) | Intra-assay precision (%) | Inter-assay precision (%) |
|---|---|---|---|---|
| ABT-888 | 10 | 100.4 | 4.5 | -* |
| 30 | 99.9 | 5.0 | -* | |
| 100 | 100.6 | 3.3 | -* | |
| 300 | 101.0 | 3.6 | 2.8 | |
| 500 | 102.4 | 2.8 | 2.0 | |
| 750 | 101.6 | 3.9 | -* | |
| 1000 | 96.5 | 3.6 | 1.7 | |
| M8 | 10 | 101.4 | 5.7 | -* |
| 30 | 97.4 | 4.2 | 2.4 | |
| 100 | 100.8 | 7.0 | -* | |
| 300 | 102.3 | 5.5 | -* | |
| 500 | 104.6 | 6.3 | -* | |
| 750 | 104.4 | 12.1 | -* | |
| 1000 | 98.3 | 11.3 | -* | |
N=9; triplicate results, each in 3 separate runs, for each concentration.
The mean square of the within runs was greater than the mean square of the between runs, indicating that there was no significant additional variation due to the performance of the assay in different runs [9].
Fig. 3.
Representative calibration curves (N=3 for each concentration) used to quantitate ABT-888 (□) and its metabolite M8 (○) in human plasma samples (response ABT-888 = 0.0187•conc − 0.0141; R2=0.9980; response M8 = 0.00675•conc − 0.0038; R2=0.9938).
3.1.3 Accuracy and precision
FDA guidelines specify that the accuracies for all tested concentrations should be within ±15%, and the precisions should not be > 15% CV except for the LLQ, in which case these parameters should not exceed 20% [8].
The accuracies and intra- and inter-assay precisions for the tested concentrations (LLQ, QCL, QCM, QCH) were all within the defined acceptance criteria (Table 2).
Table 2.
Assay performance data for the quantitation of LLQ, QCL, QCM and QCH ABT-888 and M8 concentrations in human plasma.
| Concentration (ng/mL) | Accuracy (%) | Intra-assay precision (%) | Inter-assay precision (%) | |
|---|---|---|---|---|
| ABT-888 | 10 (LLQ) | 98.2 | 4.7 | 4.9 |
| 20 (QCL) | 98.4 | 3.6 | 0.1 | |
| 200 (QCM) | 98.5 | 3.2 | 2.3 | |
| 800 (QCH) | 95.5 | 3.1 | 4.0 | |
| M8 | 10 (LLQ) | 100.9 | 7.3 | 13.7 |
| 20 (QCL) | 96.9 | 6.6 | 6.5 | |
| 200 (QCM) | 96.9 | 4.3 | 4.4 | |
| 800 (QCH) | 91.4 | 5.6 | -* | |
N=24; 8-fold results, each in 3 separate runs, for each concentration.
The mean square of the within runs was greater than the mean square of the between runs, indicating that there was no significant additional variation due to the performance of the assay in different runs [9].
3.1.4 Selectivity and specificity
According to FDA guidelines, the signal at the LLQ must be at least 5 times the signal of any co-eluting peaks [8].
Chromatograms of six individual control plasma samples contained no co-eluting peaks >20% of the analyte areas at the LLQ concentration (Fig. 2). In subsequent analyses, there were no interfering or co-eluting peaks.
3.1.5 Extraction recovery and ion-suppression
FDA-guidelines require that recovery be consistent and precise [8]. A recovery of ≥70% with a variation of 15% is generally accepted [8,9]. There is no specific guideline for the percentage of ion-suppression that is acceptable. Ultimately, the assay performance, as expressed in the precision and accuracy, is most relevant; however, a large and/or variable ion-suppression may explain an unsatisfactory assay performance. The recoveries of at the three QC concentrations ranged from 71.4 to 79.0%, with CVs between 7.3 and 21.1% (ABT-888), and from 62.2 to 67.3%, with CVs between 6.7 and 22.6% (M8). Ion-suppression ranged from 2.9 to 23.1%, with CVs between 6.1 and 14.1% (ABT-888), and from 12.3 to 26.4%, with CVs between 7.8 and 8.8% (M8) (Table 3). Although recovery was below 70% for M8 and some of the values for CVs were higher than 15%, we feel the assay had a satisfactory performance. For calculation of these parameters, absolute values were taken. After correction by the internal standard response, variability decreased substantially, and the assay performance was within the acceptable range.
Table 3.
Recoveries of ABT-888 and M8 from human plasma and their respective ion suppressions in human plasma extract, with coefficients of variation (CV).
| Concentration (ng/mL) | Recovery (%) | CV (%) | Ion suppression (%) | CV (%) | |
|---|---|---|---|---|---|
| ABT-888 | 20 (QCL) | 71.4 | 15.1 | 23.1 | 14.1 |
| 200 (QCM) | 76.0 | 7.3 | 2.9 | 6.1 | |
| 800 (QCH) | 79.0 | 21.1 | 11.5 | 9.7 | |
| M8 | 20 (QCL) | 67.3 | 12.3 | 26.4 | 8.8 |
| 200 (QCM) | 62.2 | 6.7 | 12.3 | 7.8 | |
| 800 (QCH) | 63.4 | 22.6 | 21.0 | 8.6 | |
N=3, for each concentration.
3.1.6 Stability
Stability in biological samples is acceptable when ≥85% of the analyte is recovered. The stabilities of the ABT-888 and M8 stock solutions at room temperature for 4 h were 101.8 and 101.0%, respectively (Table 4). Stabilities in stock solutions for 3 months at 4 °C were 104.9 and 98.2% for ABT-888 and M8, respectively. The stabilities of ABT-888 and M8 in plasma during freeze-thaw cycling and in plasma at room temperature (>94.4% after 4 h) were also acceptable. Long-term stabilities of ABT-888 and M8 in plasma at −80 °C were adequate with recoveries between 89.2 and 113.9%. The absolute responses of plasma extracts of ABT-888 at the calibration concentrations, when reconstituted and kept in the autosampler for 72 h, were 97.7 to 110.2% of the initial responses (CV 7.4–16.0%), while the response of ABT-888 relative to the internal standard signal ranged from 96.8 to 107.4% (CV 1.3–7.3%). The absolute responses of plasma extracts of M8 at the calibration concentrations, when reconstituted and kept in the autosampler for 72 h, were 102.7 to 121.1% of the initial responses (CV 3.6–14.5%), while the response of M8 relative to the internal standard signal ranged from 104.7 to 118.0% (CV 1.0–12.8%). The somewhat high M8 stability of 118.0% at 30 ng/mL was due to a single sample which had a 2-fold higher absolute response relative to the other samples.
Table 4.
Stability of ABT-888 and M8 under varying conditions.
| Storage condition | Concentration (ng/mL) | Stability (%) | CV (%) | Replicates | |
|---|---|---|---|---|---|
| ABT-888 | |||||
| Stock solution 4 h Ambient temp. |
1,000,000 | 101.8 | 4.0 | 3 | |
| Stock solution 3 months 4 °C |
1,000,000 | 104.9 | 2.9 | 3 | |
| Plasma 4 h Ambient temp. |
QCL | 20 | 106.5 | 6.7 | 3 |
| QCM | 200 | 95.7 | 7.0 | 3 | |
| QCH | 800 | 101.9 | 7.5 | 3 | |
| Plasma 3 freeze-thaw cycles −80 °C |
QCL | 20 | 102.1 | 2.5 | 3 |
| QCM | 200 | 98.9 | 4.9 | 3 | |
| QCH | 800 | 100.0 | 4.4 | 3 | |
| Plasma 3 months −80 °C |
QCL | 20 | 113.9 | 4.3 | 3 |
| QCM | 200 | 98.2 | 6.2 | 3 | |
| QCH | 800 | 98.5 | 4.2 | 3 | |
| M8 | |||||
| Stock solution 4 h Ambient temp. |
1,000,000 | 101.0 | 3.5 | 3 | |
| Stock solution 3 months 4 °C |
1,000,000 | 98.2 | 5.8 | 3 | |
| Plasma 4 h Ambient temp. |
QCL | 20 | 105.0 | 12.5 | 3 |
| QCM | 200 | 94.4 | 8.1 | 3 | |
| QCH | 800 | 99.9 | 6.6 | 3 | |
| Plasma 3 freeze-thaw cycles −80 °C |
QCL | 20 | 102.8 | 13.9 | 3 |
| QCM | 200 | 94.4 | 5.1 | 3 | |
| QCH | 800 | 96.8 | 4.3 | 3 | |
| Plasma 3 months −80 °C |
QCL | 20 | 90.5 | 15.6 | 3 |
| QCM | 200 | 92.7 | 12.2 | 3 | |
| QCH | 800 | 89.2 | 9.8 | 3 | |
3.1.7 Parallelism
The mean accuracy of the diluted samples was 101.9%, with a CV of 8.3% for ABT-888, and 99.8%, with a CV of 4.8%, for M8, indicating parallelism for this assay.
3.2 Application of the assay
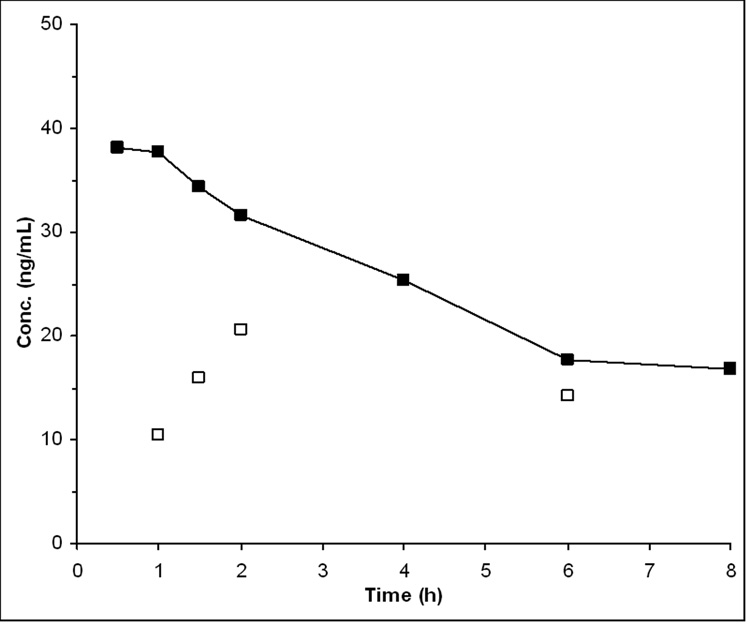
We applied the assay to samples obtained from a patient receiving a 10 mg oral dose of ABT-888, which is the lowest dose level of an ongoing phase I study. The assay was capable of quantitating ABT-888 concentrations in all samples of this patient, while M8 concentrations were above the LLOQ in most samples (Fig. 4).
Fig. 4.
Plasma concentrations of ABT-888 (■) and its metabolite M8 (□) after administration of 10 mg of ABT-888 p.o. to a patient with lung cancer enrolled in a phase I trial.
3.3 Method development
Acetonitrile precipitation resulted in a higher background signal, whereas ethyl acetate liquid-liquid extraction resulted in a lower background with comparable intensities of the analyte signals. The extraction recovery of 60–80% was sufficiently high to yield good assay performance. We did not attempt to increase recovery, because this would likely also have increased the amount of co-extracted matrix constituents. Initially, we employed an isocratic system with the same solvents, but could not resolve ABT-888 and its metabolite. Therefore, a gradient system was developed as described above, which resulted in adequate resolution. Attempts to decrease the run-time by increasing the slope of the gradient resulted in inadequate separation of the analytes from each other and matrix components. The wash-step between 10 and 25 min was required to elute matrix components that would interfere with appropriate analysis of consecutive samples (see peaks eluting between 12 and 18 min). To ensure adequate re-equilibration of the column to the initial condition of only 2% solvent A, a 10 min re-equilibration step was employed.
4 Conclusion
Our objective was to develop and validate an analytical method for the quantitation of ABT-888 and its inactive metabolite M8 in human plasma. We accomplished this using reversed phase chromatography equipped with single quadrupole mass spectrometric detection.
The method presented here allows the quantitation of ABT-888 and M8 in human plasma and, to our knowledge, is the first validated assay for ABT-888 and M8 published to date. Rodriguez et al. reported concentrations of ABT-888 in plasma and tumor, however, they did not provide any experimental details or assay parameters [10]. Using our assay, validated according to the FDA guidelines for bioanalytical method validation [8], we were able to quantitate ABT-888 and M8 in the plasma of a patient after treated orally with the lowest dose of ABT-888 that will be administered to humans.
The analytical method presented in this paper will be a valuable tool in quantitating ABT-888 and M8 plasma concentrations as ABT-888 undergoes full clinical development in combination with a variety of DNA-damaging agents.
Acknowledgements
Supported by grants UO1CA099168 and 2P30CA47904, and NIH/NCRR/CTSA Grant UL1 RR024153 from the NIH. We thank the University of Pittsburgh Cancer Institute Hematology/Oncology Writing Group for constructive suggestions regarding the manuscript, and Susan Christner for analytical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curtin NJ. Expert Rev Mol Med. 2005;7:1–20. doi: 10.1017/S146239940500904X. [DOI] [PubMed] [Google Scholar]
- 2.Low J. Solicitation for preclinical studies ( ABT-888 ( PARP inhibitor) CTEP rapid communication. 2005 [Google Scholar]
- 3.Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL, Dillehay LE, Ferguson DC, Ghoreishi-Haack NS, Grimm DR, Guan R, Han EK, Holley-Shanks RR, Hristov B, Idler KB, Jarvis K, Johnson EF, Kleinberg LR, Klinghofer V, Lasko LM, Liu X, Marsh KC, McGonigal TP, Meulbroek JA, Olson AM, Palma JP, Rodriguez LE, Shi Y, Stavropoulos JA, Tsurutani AC, Zhu GD, Rosenberg SH, Giranda VL, Frost DJ. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 4.Giranda VL, Liu J, Bauch J, Mandli M, Roberts E, Masse S, Healen-Greenberg C, Morfitt S, Marsh K, Beconi M. Proc Am Assoc Cancer Res. 2007;48:368. [Google Scholar]
- 5.Horton TM, Zhang L, Jenkins GN, Berg SL, Blaney SM. Proc Annu Meet Am Assoc Cancer Res. 2007;25:533s. [Google Scholar]
- 6.Kummar S, Kinders R, Gutierrez M, Rubinstein L, Parchment RE, Phillips LR, Low J, Murgo AJ, Tomaszewski JE, Doroshow JH. Proc Annu Meet Am Assoc Cancer Res. 2007;25:142s. [Google Scholar]
- 7.Liu SK, Coakley C, Bristow R. Int J Radiat Oncol Biol Phys. 2007;69:s615. [Google Scholar]
- 8.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Veterinary Medicine (CVM) Guidance for Industry - Bioanalytical Method Validation. 2001 http://www.fda.gov/cder/guidance/4252fnl.pdf.
- 9.Rosing H, Man WY, Doyle E, Bult A, Beijnen JH. J Liq Chromatogr Relat Technol. 2000;23:329–354. [Google Scholar]
- 10.Rodriguez LE, Palma JP, Jarvis K, Bontcheva-Diaz V, Stavropoulos J, Bukofzer G, Godzicki L, Colon-Lopez M, Saltarelli M, Liu X, Shi Y, Guan R, Luo Y, Olson A, Bouska J, Zhu G, Penning T, Giranda VL, lesniewski R, Rosenberg S, Frost DJ, Donawho CK. Proc Am Assoc Cancer Res. 2007;48:1170–1171. [Google Scholar]