Abstract
Prenatal alcohol exposure (AE) is associated with lasting abnormalities of sleep and motor development, but the underlying mechanisms are unknown. We hypothesized that AE alters development of GABAergic signaling in the hypothalamic regions important for the control of sleep and motor activity. Alcohol (5.25 g/kg/day) was administered intragastrically to male rats on postnatal days (PD) 4–9, a period of brain development equivalent to the human third trimester (AE group). Control pups were sham-intubated (S group). Motor activity was monitored on PD27–28. On PD29–30, GABAA receptor subunit mRNA levels and α4 and δ subunit proteins were quantified by RT-PCR and immunoblotting, respectively, in the wake- and motor activity-promoting perifornical (PF) region of the posterior hypothalamus and the sleep-promoting ventrolateral preoptic (VLPO) region of the anterior hypothalamus. Then, in 47–52-day old rats, motor activity was quantified following administration of GABAA receptor agonist, gaboxadol (5 mg/kg s.c.). In the PF region, mRNA and protein levels for the α4 and δ subunits were significantly higher and β3 and γ2 subunit mRNAs were also increased in the AE group. In the VLPO region, only the δ subunit mRNA was increased. Spontaneous motor activity was lower and suppressed more by gaboxadol in the AE than S group, and the latency to a transient total loss of activity after gaboxadol was shorter in the AE group. Thus, perinatal AE leads to GABAA receptor overexpression in the vigilance- and motor activity-promoting hypothalamic PF region, with the neurochemical and functional outcomes lasting long beyond the period of the insult.
Keywords: development, ethanol, GABA, hypothalamus, motor activity, sleep
Introduction
Alcohol (ethanol) consumption during pregnancy has grave and multifaceted consequences for child development, such as the fetal alcohol syndrome and related neurodevelopmental disorders collectively referred to as the fetal alcohol spectrum disorders (FASD) (reviewed in [25]). Among those, abnormal sleep patterns that correlate with impairments of motor and cognitive development during the first months of life have been observed in infants [7, 13, 15]. When tested in animal models, sleep abnormalities can persist through adulthood [8, 33, 35], but the neurochemical mechanisms underlying the disrupted development and control of sleep caused by prenatal alcohol exposure (AE) have not been elucidated.
One well documented target of AE in the developing brain is the signaling mediated by gamma-aminobutyric acid (GABA) type A receptors (GABAAR) (e.g., [2, 4,14, 29, 31]). GABAARs are pentamers that are assembled from at least 19 subunits known to date (reviewed in [30]). Different combinations of subunits result in functional receptors with different pharmacology, including responses to alcohol [30, 34, 40].Importantly, GABAARs also play a key role in the regulation of sleep. Sleep-promoting GABAergic neurons are clustered within the ventrolateral preoptic area (VLPO) of the anterior hypothalamus and innervate multiple wake-promoting brain sites including the perifornical (PF) region of the posterior hypothalamus that has wake- and motor activity enhancing functions [19, 22, 32, 36]. Activation of GABAARs in the PF region promotessleep [1, 27]. In rats, hypothalamic mechanisms start playing a major role in the consolidation of sleep between postnatal day (PD) 2 and 8 [17], and hypothalamic GABA concentrations are dramatically increased between PD 5 and 10 [11]. This period of brain growth spurt in rats is equivalent to the period of human brain development during the third trimester of pregnancy [9]. The findings that neurons of the hypothalamic circadian clock are strongly affected in a rat model of AE at this age [3, 10] also suggest that the hypothalamic regulation of sleep and motor activity is vulnerable to alcohol during this period. We hypothesized that exposure to alcohol during the brain growth spurt equivalent to the third trimester of human gestation may lead to a long-lasting upregulation of GABAA receptor function in the hypothalamic sleep- and motor activity controlling sites, and that this may be associated with a long-lasting increase in sensitivity to sedative and hypnotic effects of GABA.
Materials and methods
We used an established rat model of prenatal AE and followed the protocol described previously in which alcohol is administered to neonatal rats via intragastric intubations [12, 16, 21, 39]. The procedures for animal handling followed the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Neonatal treatments were administered to male, Sprague-Dawley rats. Seven litters from timed-pregnant rats were culled and cross-fostered among lactating dams to bring each litter size to 8–10 pups. On PD4, the pups within each litter were randomly assigned to either alcohol- (AE) or sham-treated (S) group. The AE group received a daily dose of 5.25 g/kg of alcohol on PD4 through 9 administered as two intragastric intubations per day (2.625 g/kg per intubation, 11.9% v/v in a custom milk formula, 2 h apart). Data indicate that such an exposure results in cognitive deficits in 30 day-old rats 5 [39]. Two hours after the second intubation, an additional intubation with milk only was administered to the AE group to offset reduced maternal milk consumption. The S group underwent the same daily routine of intubations, but no fluid was infused [12, 16, 39]. Pups were weighed daily (Fig. 1A) and stayed with the dam until weaned on PD21–22. Blood was collected on PD4 from a separate group of animals (n=7) sacrificed 2 h after the second alcohol intubation, and blood alcohol concentration (BAC) was determined using NAD-ADH reagent (Sigma, USA).
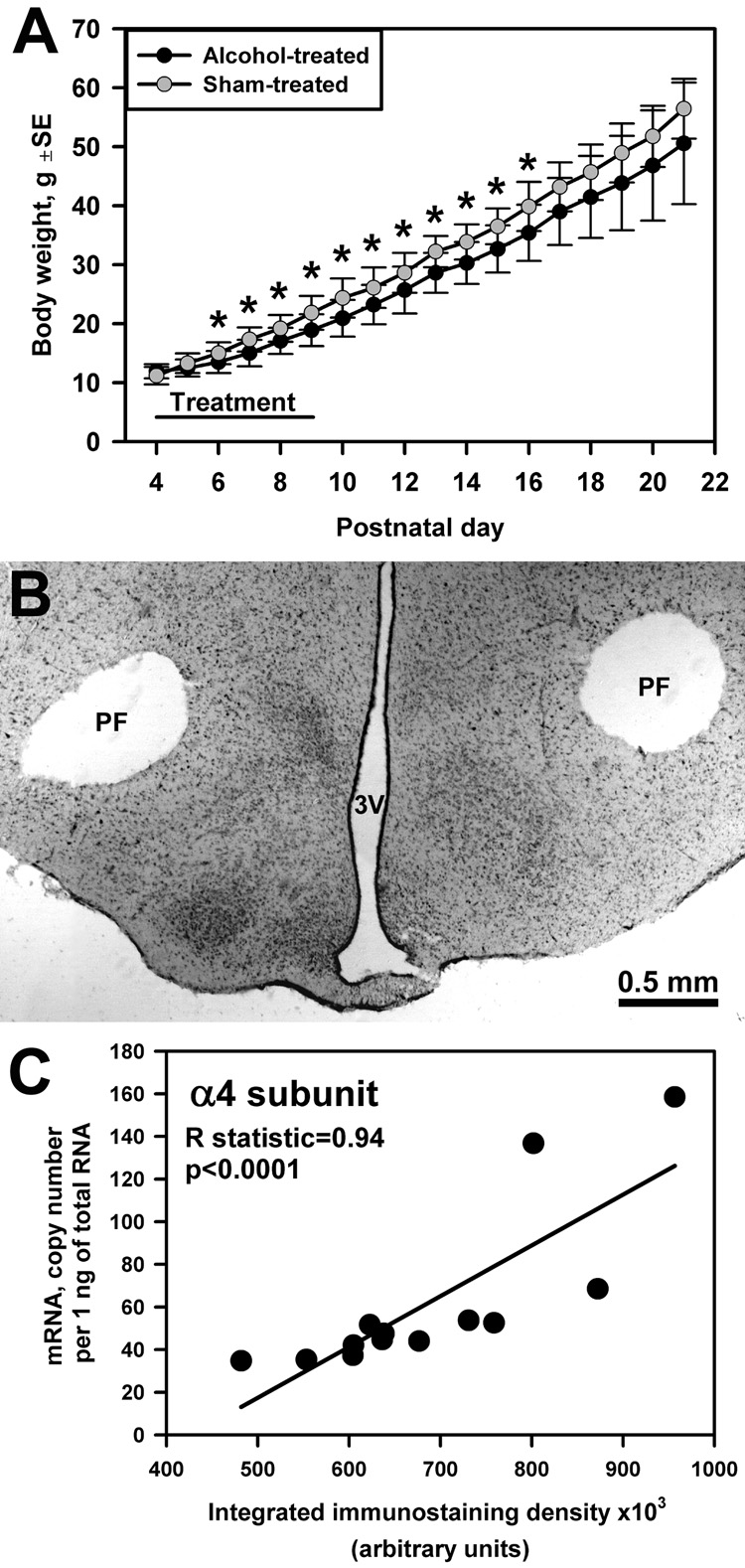
Fig. 1.
(A) Group mean body weight in alcohol- and sham-treated rats (n=11–23 per group) from the beginning of treatment on PD4 to weaning (*, p<0.05 between groups). (B) Neutral red-stained section from a posterior hypothalamic slice showing the location of 700 µm tissue micropunches taken bilaterally from the PF region (PF) (3V, third ventricle). (C) Positive correlation between integrated density of α4 subunit dot-blot immunostaining in hypothalamic tissue samples extracted from one side of the slices containing the PF or VLPO region and α4 subunit mRNA levels in samples taken from the opposite side of each slice (data from 13 pairs of samples from 7 sham-treated rats).
On PD29–30, 500 µm-thick hypothalamic slices were obtained from rats sacrificed at a constant circadian time (2–4 pm). Two pairs of tissue micropunches, 700 µm in diameter, were cut bilaterally, one from the PF region of the posterior hypothalamic slice (Fig. 1B) and the other from the VLPO region of the anterior hypothalamic slice. One of the samples from each pair was subjected to RNA extraction and quantitative reverse transcription-polymerase chain reaction (RT-PCR) using the protocol described in our earlier study [38]. We quantified mRNA levels for eleven subunits of GABAAR (α1–5, β1–3, γ2 (long and short splicing variants; γ2L/S), δ, and ε) that are expressed in the hypothalamus [24]. The other sample was frozen on dry ice and stored at −80°C for protein immunodetection. Each slice was then fixed in formalin and cut into 30 µm thick sections that were subsequently stained with Neutral red to verify the micropunch location (Fig. 1B).
GABAAR subunit-like protein levels for α4 or δ subunits were quantified using dot-blot immunodetection [43]. The starting amount of tissue required for the assay is much smaller than in any other immunoblotting technique, which allowed us to quantify both mRNA and protein in symmetrically located tissue micropunches of the same size. Samples were solubilized and 0.5 µl of the crude extract was absorbed onto a nitrocellulose membrane. All samples from both groups were placed together on one membrane, and then sequentially incubated in primary antibodies against either α4 (Affinity BioReagents, USA; OPA1-04102, 1:1,000) or δ subunit (Chemicon, USA; AB9752, 1:1,000), biotinylated secondary antibodies, avidin-horseradish peroxidase complex (ABC kit, Vector, USA), and then visualized with diaminobenzidine with nickel ammonium sulphate. The images of membranes were digitized and immunostaining density was quantified using ImageJ software (National Institutes of Health, http://rsb.info.nih.gov). Quality controls included histological verification of punch locations and assessment of RNA and PCR quality, as described previously [38].
On PD27–28 and then on PD47, basal motor activity was monitored during the lights-on (rest) period (11:00 am – 2:00 pm) using a system of infrared light beams (AccuScan Instruments, USA). During each session, two rats, one from the AE and one from S group, were tested simultaneously in their separate home cages. Each recording session lasted 90 min, with the number of separate movements averaged over successive 30-min intervals using the software supplied by the manufacturer. On PD51–52, using the same recording setup, we assessed the effect of GABAA agonist, gaboxadol, whose effects are mediated by α4- and δ subunit-containing GABAARs [5, 26, 41]. Animals from both groups were tested in pairs following s.c. administration of gaboxadol (THIP, Sigma; 5 mg/kg [20]) dissolved in isotonic saline at the beginning of the recording session [5].
The significance of differences between the AE and S groups was examined using one-way ANOVA with Bonferroni’s correction (Analyse-It, UK). The differences were considered significant when P<0.05.
Results and discussion
Mean BAC in AE rats was 351±24 (SE) mg/dl, consistent with previously published results obtained from a similar experimental design [14, 16, 21]. Body weight gains were significantly lower in pups of the AE than S group on PD6 through 16 (Fig. 1A) reflecting a growth lag similar to that described in earlier studies [16, 18, 21, 39]. This difference was not significant on PD29–30 (100±4 (SE) g in AE vs. 107±3 in S, p=0.16, n=12). The difference in body weight gain during the treatment was probably caused by reduced caloric intake due to inhibited suckling in AE rats because feeding with a high-caloric fat emulsion instead of balanced milk formula eliminates this difference [18]. Since we used a mixed litter design, we cannot exclude a possibility of growth disadvantage of AE pups resulting from a selectively altered maternal interaction.
The integrated density of GABAAR subunit immunostaining was positively correlated with mRNA levels measured in symmetrically located samples obtained from the same slices (R=0.94; p<0.0001 for the α4 subunit (Fig. 1C); and R=0.59; p<0.04 for the δ subunit (not shown); n=13 sample pairs from either PF or VLPO region; Spearman rank correlation).
When quantified on PD29–30, 19 days after the last AE, mRNA levels for the α4, β3, γ2L/S and δ subunits of GABAAR were significantly higher in the PF region in the AE than the S group. In contrast, in the VLPO region, only the δ subunit mRNA was higher in the AE than the S group (Table 1).
Table 1.
Mean GABAA receptor subunit mRNA levelsa measured in the perifornical (PF) and ventrolateral preoptic (VLPO) regions in alcohol- and sham-treated rats on postnatal day 29–30.
| GABAAR subunit mRNA |
Alcohol-treated | Sham-treated | ||
|---|---|---|---|---|
| PF | VLPO | PF | VLPO | |
| (n=6) | (n=6 or 7) | (n=7) | (n=6 or 7) | |
| α1 | 16,000±1,100 | 18,400±1,300 | 14,000±1,100 | 18,300±2,200 |
| α2 | 8,500±400 | 7,700±600 | 8,100±800 | 7,100±1,200 |
| α3 | 10,700±600 | 10,500±1,100 | 10,400±700 | 9,400±500 |
| α4 | 61±6 * | 76±10 | 42±3 | 88±17 |
| α5 | 5,000±600 | 2,300±50 | 4,800±600 | 2,300±190 |
| β1 | 10,000±1,100 | 6,500±200 | 9,700±1,100 | 7,200±900 |
| β2 | 24,000±3,700 | 26,000±1,500 | 19,000±1,700 | 26,000±2,900 |
| β3 | 84,000±12,200 * | 66,000±4,000 | 58,000±2,200 | 66,000±7,000 |
| γ2 | 580±37 * | 1,800±280 | 450±20 | 1,800±500 |
| δ | 2.46±0.34 * | 49±11 * | 1.6±0.2 | 21±4 |
| ε | 150±14 | 27±5 | 150±30 | 36±4 |
Expressed as cDNA copy numbers per 1 ng of total RNA in tissue sample ± SE.
p<0.05 for differences between alcohol- and sham-treated rats.
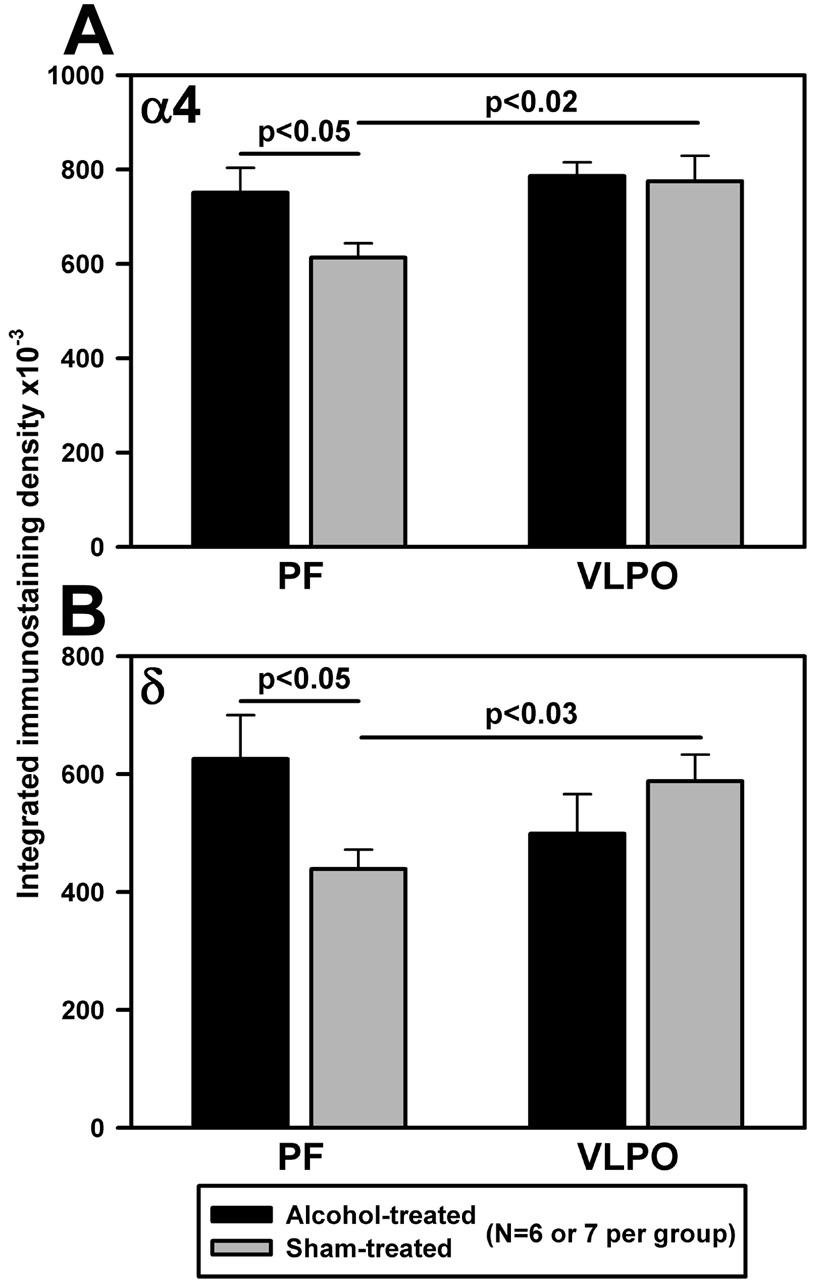
Immunoreactivity for both α4 and δ subunits also was significantly increased in the PF region in the AE compared to the S group on PD29–30, whereas the levels of these subunits were not significantly different between the two groups in the VLPO region (Fig. 2).
Fig. 2.
Neonatal exposure to alcohol leads to increased immunoreactivity for α4 (A) and δ (B) subunits of GABAA receptor in the PF region in 29–30 day-old rats.
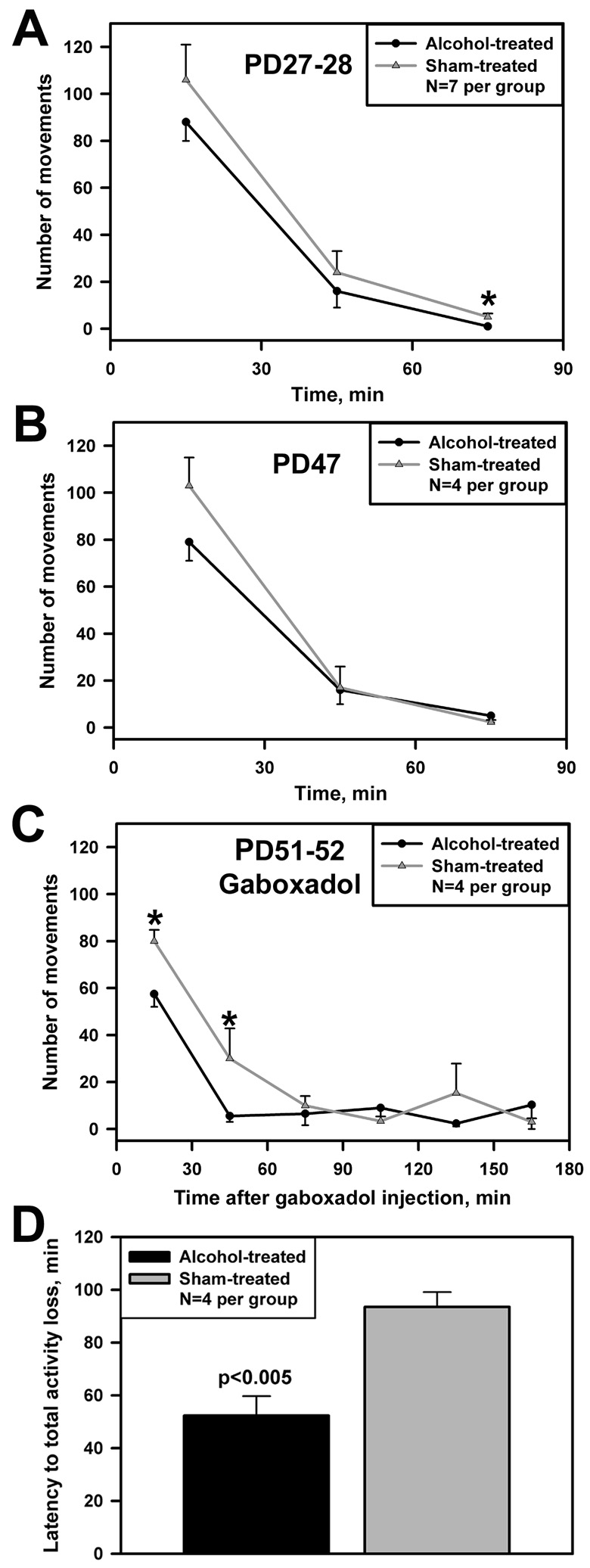
On PD27–28, 17 days after the last AE, the number of spontaneous movements was significantly lower in the last 30-min interval in rats subjected to AE when compared to the S group (p<0.02; Fig. 3A). During the first 60 min of recording, rats from both groups had high levels of motor activity, probably due to novelty of the environment, but the number of movements also tended to be lower in the AE than S group.
Fig. 3.
Neonatal exposure to alcohol results in reduced spontaneous motor activity in 27–28 day-old rats (A), with the same trend also present on PD47 (B) (*, p<0.05 between groups). Following gaboxadol treatment (5 mg/kg, s.c.) on PD51–52, motor activity is reduced more profoundly (C), and the latency to gaboxadol-induced transient period of immobility lasting at least 3 min is shorter in the alcohol- than sham-treated rats (D).
On PD47, 37 days after the last AE, the number of spontaneous movements also tended to be lower in the AE group (Fig. 3B). Then, on PD51–52, administration of gaboxadol caused a significantly larger decline of the number of movements in the AE than the S group (Fig. 3C). In rats from both groups, gaboxadol caused a transient total loss of motor activity, consistent with its sedative and sleep-promoting properties described previously [20]. The latency to the first period of immobility lasting over 3 min was significantly shorter in the AE than the S rats (p<0.005; Fig. 3D).
Interruption of the normal time course of GABAergic system development may disrupt trophic influences of GABA and maturation of neuronal circuits (e.g., [23]). Data also indicate that prenatal AE can cause an increase in the number of cortical GABAARs lasting through adulthood [4]. Similarly, we found that both mRNA and protein levels for the α4 and δ subunits and mRNA levels for two additional subunits were elevated in young adult rats subjected to perinatal AE. Importantly, the changes were region-specific in that they occurred in the PF region but were less evident in the VLPO region. A proportional relationship between mRNA levels and immunoreactivity for the α4 and δ subunits suggested that both transcriptional and translational mechanisms were involved in upregulation of these subunits in rats subjected to perinatal AE. Since the posterior hypothalamus also regulates food intake, it seems possible that regional molecular changes can be caused by reduced caloric intake during the AE. It remains uncertain though whether GABAergic system can be affected. Data from adult rats indicate that caloric deprivation does not alter hypothalamic mRNA levels for GABAergic precursor, glutamic acid decarboxylase, whereas neuropeptide Y mRNA is elevated [28].
A number of previous studies reported an increase in the brain α4 subunit expression following acute or chronic AE, both in vivo and in vitro (reviewed in [42]). Data show that the α4-β3-δ subunit combination can create a GABAAR that is extremely sensitive to alcohol at low concentrations [34, 40], although in a recent study, the uniquely high sensitivity of such recombinant receptors to alcohol has been questioned [6]. The GABAARs containing these subunits are often extrasynaptic and mediate tonic inhibition [5, 26, 30, 40]. Recent data indicate that extrasynaptyc GABAARs containing the putative alcohol-sensitive combinations, α4 (or α6) and δ subunits, mediate effects of gaboxadol, a sleep-promoting GABAA agonist [5, 26, 41], and that β3 subunit is also important for the regulation of sleep [37]. Of these subunits, the α6 subunit was not investigated in our study because it is very weakly expressed in the hypothalamus [24].
Thus, perinatal AE results in long-lasting molecular changes in the arousal- and motor activity promoting PF region that can enhance GABAergic inhibition exerted in this region. Our functional data indicating that motor activity is reduced in AE rats may be explained by overexpression of posterior hypothalamic GABAARs. Since upregulation of GABAARs occurs in the PF region when sleep drive is increased [37], reduced motor activity in the AE rats suggests that their sleep and sleepiness are also increased. Our results demonstrate that AE rats have reduced spontaneous motor activity 17 days after AE and exhibit the same trend 37 days after the insult. In addition, gaboxadol administered 41 days after AE suppressed motor activity more powerfully in the AE group, and AE rats developed immobility more rapidly than the rats of the S group. This is consistent with the possibility of increased inhibition mediated by α4 and δ subunit containing GABAARs because we found that both mRNA and protein levels for these subunits were elevated in the arousal- and motor activity-promoting PF region in the AE rats, and gaboxadol can act preferentially through GABAARs that include these subunits [5, 26, 41]. These changes can underlie an impaired regulation of motor activity and sleep-wake behavior that last long beyond the period of the perinatal exposure to alcohol.
Acknowledgments
The study was supported by the American Sleep Medicine Foundation award 26-CA-04 and NIH Grant HL-071097. The author thanks Dr. Leszek Kubin for support and discussions, and Ms. Tyana Singletary for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alam M, Kumar S, Bashir T, Suntsova N, Methippara M, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan A, Wu H, Paxton L, Savage D. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acid A1 receptor-gated chloride ion channel in adult rat offspring. J Pharmacol Exp Ther. 1998;284:250–257. [PubMed] [Google Scholar]
- 3.Allen GC, West JR, Chen WJA, Earnest DJ. Developmental alcohol exposure disrupts circadian regulation of BDNF in the rat suprachiasmatic nucleus. Neurotoxicol and Teratol. 2004;26:353–358. doi: 10.1016/j.ntt.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey CDC, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure increases GABAA receptor subunit protein expression in the adult guinea pig cerebral cortex. J Neurosci. 2001;21:4381–4389. doi: 10.1523/JNEUROSCI.21-12-04381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehm SL, II, Homanics G, Blednov Y, Harris R. δ-Subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-ol. Eur J Pharmacol. 2006;541:158–162. doi: 10.1016/j.ejphar.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 6.Borghese C, Harris R. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Angiulli A, Grunau P, Maggi S, Herdman A. Electroencephalographic correlates of prenatal exposure to alcohol in infants and children: a review of findings and implications for neurocognitive development. Alcohol. 2006;40:127–133. doi: 10.1016/j.alcohol.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 8.De A, Mukherjee S, Krueger JM, Simasko SM. Prenatal ethanol exposure changes non rapid eye movement sleep in adult male rats. Sleep. 2005;28:A31–A31. [Google Scholar]
- 9.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 10.Farnell YZ, West JR, Chen WJA, Allen GC, Earnest DJ. Developmental alcohol exposure alters light-induced phase shifts of the circadian activity rhythm in rats. Alcohol Clin Exp Res. 2004;28:1020–1027. doi: 10.1097/01.alc.0000130807.21020.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flugge G, Wuttke W, Fuchs E. Postnatal development of transmitter systems - sexual-differentiation the GABAergic system and effects of muscimol. Intl J Dev Neurosci. 1986;4:319–326. doi: 10.1016/0736-5748(86)90049-3. [DOI] [PubMed] [Google Scholar]
- 12.Goodlett C, Johnson T. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- 13.Havlicek V, Childiaeva R, Chernick V. EEG frequency spectrum characteristics of sleep states in infants of alcoholic mothers. Neuropediatrics. 1977;8:360–373. doi: 10.1055/s-0028-1091532. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao SH, Parrish AR, Nahm SS, Abbott LC, McCool BA, Frye GD. Effects of early postnatal ethanol intubation on GABAergic synaptic proteins. Dev Brain Res. 2002;138:177–185. doi: 10.1016/s0165-3806(02)00470-4. [DOI] [PubMed] [Google Scholar]
- 15.Ioffe S, Chernick V. Prediction of subsequent motor and mental retardation in newborn infants exposed to alcohol in utero by computerized EEG analysis. Neuropediatrics. 1990;21:11–17. doi: 10.1055/s-2008-1071450. [DOI] [PubMed] [Google Scholar]
- 16.Johnson T, Goodlett C. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcohol Clin Exp Res. 2002;26:83–93. [PubMed] [Google Scholar]
- 17.Karlsson K, Kreider J, Blumberg M. Hypothalamic contribution to sleep-wake cycle development. Neuroscience. 2004;123:575–582. doi: 10.1016/j.neuroscience.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Light K, Kane C, Pierce D, Jenkins D, Ge Y, Brown G, Yang H, Nyamweya N. Intragastric intubation: important aspects of the model for administration of ethanol to rat pups during the postnatal period. Alcohol Clin Exp Res. 1998;22:1600–1606. doi: 10.1111/j.1530-0277.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Greco M, Shiromani P, Saper C. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Scnchez C, Saper C, Vogel V. Soc Neurosci, Program No 617.1. Washington, DC: 2003. Gaboxadol activates endogenous sleep control mechanism. Abstract Viewer/Itinerary Planner. [Google Scholar]
- 21.O'Leary-Moore S, McMechan A, Mathison S, Berman R, Hannigan J. Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol. 2006;38:99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 24.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 25.Riley E, McGee C. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 26.Saarelainen K, Ranna M, Rabe H, Sinkkonen S, Möykkynen T, Uusi-Oukari M, Linden A, Lüddens H, Korpi E. Enhanced behavioral sensitivity to the competitive GABA agonist, gaboxadol, in transgenic mice over-expressing hippocampal extrasynaptic α6β GABAA receptors. J Neurochem. 2008;105:338–350. doi: 10.1111/j.1471-4159.2007.05136.x. [DOI] [PubMed] [Google Scholar]
- 27.Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–683. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz M, Sipols A, Grubin C, Baskin D. Differential effect of fasting on hypothalamic expression of genes encoding neuropeptide Y, galanin, and glutamic acid decarboxylase. Brain Res Bull. 1993;31:361–367. doi: 10.1016/0361-9230(93)90228-4. [DOI] [PubMed] [Google Scholar]
- 29.Sebe J, Eggers E, Berger A. Differential effects of ethanol on GABAA and glycine receptor-mediated synaptic currents in brain stem motoneurons. J Neurophysiol. 2003;90:870–875. doi: 10.1152/jn.00119.2003. [DOI] [PubMed] [Google Scholar]
- 30.Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- 31.Spear LP, Penson J, Linville DG. GABA and behavioral inhibition in the neonatal rat pup. Psychopharmacol (Berl) 1986;90:106–111. doi: 10.1007/BF00172880. [DOI] [PubMed] [Google Scholar]
- 32.Steininger T, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol. 2001;429:638–653. [PubMed] [Google Scholar]
- 33.Stone W, Altman H, Hall J, Arankowsky-Sandoval G, Parekh P, Gold P. Prenatal exposure to alcohol in adult rats: relationships between sleep and memory deficits, and effects of glucose administration on memory. Brain Res. 1996;742:98–106. doi: 10.1016/s0006-8993(96)00976-6. [DOI] [PubMed] [Google Scholar]
- 34.Sundstrom-Poromaa I, Smith D, Gong Q, Sabado T, Li X, Light A, Wiedmann M, Williams K, Smith S. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sylvester L, Kapron C, Smith C. In utero ethanol exposure decreases rapid eye movement sleep in female Sprague-Dawley rat offspring. Neurosci Lett. 2000;289:13–16. doi: 10.1016/s0304-3940(00)01261-1. [DOI] [PubMed] [Google Scholar]
- 36.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 37.Volgin D, Kubin L. GABAA receptor subunit mRNAs are differentially regulated in the hypothalamic perifornical region in association with sleep loss and circadian time. Sleep. 2003;26 Suppl:A38–A39. [Google Scholar]
- 38.Volgin DV, Kubin L. Regionally selective effects of GABA on hypothalamic GABAA receptor mRNA in vitro. Biochem Biophys Res Comm. 2007;353:726–732. doi: 10.1016/j.bbrc.2006.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner A, Hunt P. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- 40.Wallner M, Hanchar H, Olsen R. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winsky-Sommerer R, Vyazovskiy V, Homanics G, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABAA δ-subunit-containing receptors. Eur J Neurosci. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- 42.Worst T, Vrana K. Alcohol and gene expression in the central nervous system. Alcohol Alcohol. 2005;40:63–75. doi: 10.1093/alcalc/agh119. [DOI] [PubMed] [Google Scholar]
- 43.Zeder-Lutz G, Cherouati N, Reinhart C, Pattus F, Wagner R. Dot-blot immunodetection as a versatile and high-throughput assay to evaluate recombinant GPCRs produced in the yeast Pichia pastoris. Protein Expr Purif. 2006;50:118–127. doi: 10.1016/j.pep.2006.05.017. [DOI] [PubMed] [Google Scholar]