Abstract
Olfactory dysfunction is common in patients with Parkinson disease (PD) and has been attributed to early pathological deposition of Lewy bodies and Lewy neurites in primary olfactory centers. However, olfactory deficits do not always worsen over time despite progression of disease raising the possibility of additional pathobiological mechanisms contributing to olfactory functions in PD, such as changes in olfactory neurotransmitter functions. Neurotransmitter changes, such as altered dopaminergic status, may also better explain the selective nature of odor identification deficits in PD. Proper odor identification depends on higher order structures, such as the hippocampus, for olfactory cognitive or memory processing. Using the University of Pennsylvania Smell Identification Test (UPSIT), we previously identified 3 odors (banana, licorice, dill pickle, labeled as UPSIT-3) that PD subjects most frequently failed to recognize compared to age- and gender matched controls. We also identified 6 odors that were equally successfully identified by controls and PD subjects (NPD-Olf6). A ratio of UPSIT-3 divided by NPD-Olf6 scores provides another descriptor of selective hyposmia in PD (“olfactory ratio”). In this study we investigated the pathophysiology of hyposmia in PD using dopamine transporter (DAT) PET. Twenty-nine PD patients (Hoehn and Yahr stages I-III; 7f/22m; age 60.2±10.8) underwent olfactory testing using the UPSIT and [11C]β-CFT DAT PET. DAT binding potentials (BP) were assessed in the hippocampus, amygdala, ventral and dorsal striatum. We found that correlation coefficients between total UPSIT scores and regional brain DAT BP were highest for the hippocampus (Rs=0.54, P=0.002) and lower for the amygdala (Rs=0.44, P=0.02), ventral (Rs=0.48, P=0.008) and dorsal striatum (Rs=0.39, P=0.03). Correlations were most significant for the selective hyposmia measures and hippocampal DAT: UPSIT-3 (Rs=0.65, P=0.0001) and the olfactory ratio (Rs=0.74, P<0.0001).
We conclude that selective hyposmia in PD is more robustly correlated with hippocampal rather than amygdala, ventral or dorsal striatal dopamine innervation as shown by DAT binding. These findings indicate that mesolimbic dopamine innervation of the hippocampus may be a determinant of selective hyposmia in PD.
Keywords: Hippocampus, amygdala, [11C]β-CFT, dopamine, smell, Parkinson disease, PET
Introduction
Olfactory dysfunction is a frequent non-motor symptom of PD that involves deficits in odor detection, discrimination and identification [27]. Hyposmia may be related to neuronal degeneration with deposition of alpha-synuclein in primary olfactory areas as a very early component of the pathology of PD [5, 17]. It should be noted that olfactory functions in PD do not have a linear relationship with disease progression with some studies showing progressive worsening, others indicating no change, and even a single study questioning possible improvement over time [9, 16, 19]. Such variable longitudinal changes may point to additional pathobiological mechanisms contributing to olfactory functions in PD, such as disease-related changes in olfactory neurotransmitter functions. Although multiple neurotransmitters are involved in olfaction, including cholinergic, noradrenergic and serotonergic systems, there is pharmacological evidence that dopaminergic modulation plays a role in altering odor detection thresholds, odor discrimination, and learning capabilities in animal studies [37]. With respect to dopamine, we and other researchers have previously reported an association between hyposmia and the degree of nigrostriatal dopaminergic denervation in PD [4, 32], but the neostriatum does not appear to have primary involvement in olfactory processing. Furthermore, impairments in odor identification in PD have been reported to be selective where specific odors may be more difficult to identify than others [4, 13, 18]. Odor identification requires recognizing or naming the odor, a learned response, and raises the possibility of altered function of structures, such as the hippocampus, involved in higher order cognitive or memory processing. A neurochemical hypothesis, such as altered dopaminergic status, might also better explain the selective nature of odor identification deficits in PD.
We conducted the present study to test the hypothesis that selective deficits in odor identification would correlate more closely with dopaminergic activity in an area related to cognitive or memory processing, the hippocampus, than other areas. We assessed DAT binding in the hippocampus, amygdala and ventral striatum and compared this to neostriatal structures to determine if extension of the dopamine denervation from the nigrostriatal to the mesolimbic, especially hippocampal, component of the ascending dopamine pathways correlates more closely with selective hyposmia in PD.
Methods
Subjects and clinical testing
The study involved 29 subjects with PD: 22 males and 7 females. Patients met the UK Parkinson's Disease Society Brain Bank Research Center clinical diagnostic criteria for PD [21]. The mean age was 60.2±10.8 years and the mean duration of disease was 2.7±3.0 years. Patients had mild to moderate severity of disease: 10 patients in stage 1, 7 patients in stages 1.5, 5 patients in stage 2, 6 patients in stage 2.5 and one patient in stage 3 of the Hoehn and Yahr classification. The mean mini-mental status examination (MMSE) score was 29.5±0.8. The mean motor Unified Parkinson’s Disease Rating Scale (UPDRS) was 15.5±8.2.
Patient and control subjects underwent olfactory testing using the UPSIT (Sensonics, Inc. Haddonfield, NJ). Using the UPSIT, we previously identified 3 odors (banana, licorice, dill pickle) out of 40 odors that were most selectively impaired in PD subjects compared to age- and gender matched controls (UPSIT-3) [4]. We also identified 6 odors for which patients had similar performance compared to controls (NPD-olf6). We then calculated a ratio of the UPSIT-3 score divided by the NPD-olf6 score (olfactory ratio) to generate another measure of selective hyposmia [4]. The Center for Epidemiologic Studies Depression Scale (CES-D) was used as a self-report assessment scale for depression [31]. All subjects were non-smokers. Nineteen patients were taking a variable combination of amantadine, selegiline, carbidopa-levodopa, or dopamine agonists. Ten patients were drug-naive. Subjects on dopaminergic drugs were examined for both motor UPDRS testing and imaging in the morning after withholding dopaminergic drugs overnight. The study was approved by the Institutional Review Board of the University of Pittsburgh.
Dopamine transporter PET and MRI Imaging
[11C]β-CFT (2-β-carbomethoxy-3β-(4-fluorophenyl) tropane) is a specific radioligand for the DAT. Dynamic [11C]β-CFT DAT PET imaging with correction for attenuation, scatter and radioactive decay and brain MR imaging using a volumetric spoiled gradient recall sequence was performed as previously reported [4]. Volumes of interest (VOIs) were drawn on the MR to include the hippocampus, amygdala and striatum (split into ventral and dorsal portions) of each hemisphere and the cerebellum. All MR-drawn VOIs were transferred to the PET data for regional sampling of radioactivity. [11C]β-CFT BP was calculated using a two-parameter multilinear reference tissue model approach (MRTM2) with the cerebellum as the reference region [23]. Three hippocampal and two amygdala BPs that were slightly negative were set to zero.
Results
The mean striatal DAT BP was 1.13±0.48. The mean value for hippocampal and amygdala DAT BP were 0.07±0.05 and 0.09±0.05, respectively. There was a significant relationship between hippocampal and amygdala DAT binding (Rs=0.57, P=0.001). Correlation coefficients between hippocampal and ventral versus dorsal striatal BP were 0.42 (P=0.02) and 0.25 (ns), respectively. Table 1 lists the Spearman rank correlation coefficients between the regional DAT binding potential and the different olfactory scores.
Table 1.
Spearman rank correlation coefficients between the regional DAT binding potential and the smell measures.
| VENTRAL STRIATUM | DORSAL STRIATUM | AMYGDALA | HIPPOCAMPUS | |
|---|---|---|---|---|
| Total UPSIT | R=0.48 (P=0.008) |
R=0.39 (P=0.03) | R=0.43 (P=0.02) | R=0.54 (P=0.003) |
| UPSIT-3 | R=0.48 (P=0.01) |
R=0.46 (P=0.01) | R=0.45 (P=0.01) | R=0.65 (P=0.0001) |
| Olfactory ratio | R=0.45 (P=0.02) |
R=0.45 (P=0.02) | R=0.45 (P=0.01) | R=0.74 (P<0.0001) |
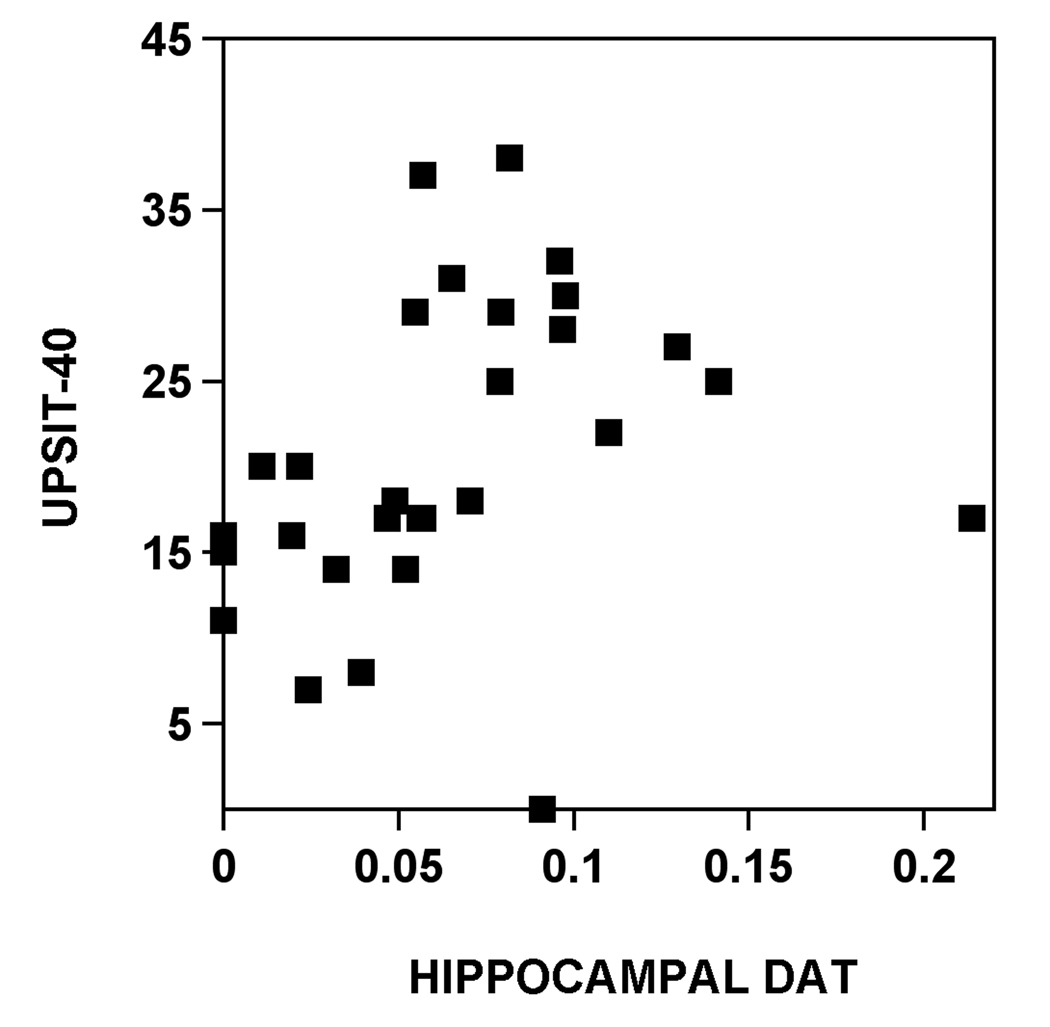
Correlation coefficients between total UPSIT scores and regional brain DAT BP were highest for the hippocampus (Figure 1), followed by the ventral striatum and lower for the amygdala and dorsal striatum.
Figure 1.
Scatter plot of the relationship between hippocampal DAT activity and total UPSIT score.
Findings were most significant for correlations with the selective hyposmia measures (UPSIT-3 and olfactory ratio) and hippocampal DAT (Table 1).
A post hoc analysis was performed to evaluate possible effects of several clinical factors. Gender-wise, both sexes demonstrated significant correlations between hippocampal DAT BP and the olfactory ratio (males, n=22, Rs=0.72, P=0.0002, and for females, n=7, Rs=0.90, P=0.006). Correlation coefficients between the olfactory ratio and hippocampal DAT in dopaminergic drug-naive (n=10) and drug non-naive patients (n=19) were Rs=0.52, P=0.14 and Rs=0.77, P=0.0001, respectively. Although there was a borderline modest correlation between hippocampal DAT and motor UPDRS scores (Rs=−0.36, P=0.06), there was no significant correlation between the olfactory ratio and motor UPDRS scores (Rs=−0.11, ns). Finally, we did not find significant correlation between scores on the depression rating scale and amygdala DAT BP (Rs=−0.07, ns) nor between depression and olfactory ratio scores (Rs=0.1, ns).
Discussion
We found that hyposmia in PD is more closely correlated with hippocampal than striatal or amygdala dopaminergic denervation, a relationship more robust for selective hyposmia than general smell identification scores. There were no significant gender differences. These findings suggest that dopamine denervation of the hippocampus may be a component of the pathophysiology of selective hyposmia in PD. Odor identification in the UPSIT is a complex task requiring peripheral as well as higher level processing and involves verbal memory or recognition within the hippocampus, prefrontal cortex, amygdala (depending on the emotional valence of the stimulus) and language areas to effectuate proper response selection [36]. Thus, olfactory identification may involve a process utilizing sensory processing in the piriform cortex and then interaction between hippocampal and prefrontal cortical mechanisms to produce a correct identification of the odor name [15, 26].
It is true that anosmia is somewhat rare in PD; even those patients most impaired in odor identification are still able to detect some strong odors, indicating that impairment in identification involves cognitive processes beyond that of detection threshold [27]. While the olfactory impairment in PD has been described in odor identification, odor discrimination, threshold detection and odor recognition memory, studies have shown that these different domains may be affected disproportionally. For example, Potagas et al. used unique tests of odor discrimination and identification to demonstrate a preferential decline in odor identification rather than odor detection, invoking impairment in odor memory [30].
Olfactory deficits may be explained by a recently proposed 6-stage schema of progressive deposition of alpha-synuclein pathology in the PD brain where the initial changes in stage 1 of the disease start simultaneously in the dorsal nucleus of the vagus nerve and olfactory nucleus and bulb [5, 17]. In this schema, however, hippocampal involvement may not be present until stage 3 or 4 and with neocortical changes occurring even later. Our findings may be in disagreement with this pathological staging system as it may imply more advanced disease associated with hyposmia in PD. It should be noted that retrospective clinico-pathologic studies, although largely confirming the staging system, particularly for younger onset PD with long duration, have identified some cases who did not follow the proposed caudo-rostral progression pattern of alpha-synuclein pathology and suggests that simultaneous involvement of subcortical and cortical regions appears to be possible [24, 25]. Furthermore, the Braak et al. staging is purely based on anatomic deposition of Lewy body or Lewy neurite pathology and does not take into account neurochemical changes. For example, a recent PET study on serotonergic denervation found preliminary evidence of a reverse caudo-rostral gradient with greater denervation present in the forebrain than in the brainstem regions in a small sample of early PD subjects [1]. It should also be noted that subtle neurocognitive deficits already may be part of the prodromal PD syndrome as neuropsychological abnormalities may be detectable in first degree relatives of patients with familial PD [14].
The mesocortical dopamine system originating in the ventral tegmental area (VTA) predominantly provides innervation to ventral striatum, basal forebrain and cortex [3]. Our findings of a more robust association between hyposmia and hippocampal rather than striatal DAT would suggest that selective hyposmia in PD is more related to dopaminergic denervation of the VTA than pure nigrostriatal denervation. This is also supported by our findings of better correlations between hippocampal and ventral compared to dorsal striatal DAT binding. The VTA, as the primary source of innervation of the mesocortical dopaminergic projections, may also be involved in compensatory mechanisms at the level of the orbitofrontal cortex in PD hyposmia as suggested by fMRI studies [38].
Further support for dopamine as a modulator of higher level olfactory processing comes from a animal study reporting olfactory discrimination deficits in mice lacking the DAT or the D2 dopamine receptor [34]. In 6-hydroxydopamine (6-OHDA) lesioned hemiparkinsonian rats, PET imaging has shown reduced DAT binding in the ipsilateral hippocampus [28], and also a significant correlation between loss of dopaminergic innervation and hippocampal neuronal glucose hypometabolism [6].
Pharmacological studies provide further evidence for a significant role for the dopamine system in olfaction, including effects on altering odor detection thresholds as well as odor discrimination and learning capabilities in animals [7, 37]. When rats were administered the dopamine D2 receptor agonist quinpirole, for example, rat odor detection performance decreased; this effect was eliminated when pre- or post-treated with the D2 antagonist spiperone, further illustrating the specificity of the effect [11]. In contrast, the administration of the D1-selective partial agonist SKF 38393 enhanced odor detection performance, whereas the D1 receptor antagonist SCH 23390 eliminated this effect [10]. Similarly, activation of D1 receptors or blockade of D2 receptors each have been shown to improve the ability of adult rats to discriminate structurally and perceptually similar odorant pairs, whereas D1 blockade or D2 activation impair odor discrimination [39].
Clinically, however, olfactory dysfunction in PD patients, does not appear to respond to dopaminergic medication [12]. The opposing effect of D1 and D2 receptor stimulation on olfactory functions might provide a possible explanation why l-DOPA does not significantly affect olfaction in PD. However, this clinical observation might also imply a neuronal population that was damaged early enough that no response would be possible by the time antiparkinsonian medications are typically prescribed [27], or alternatively, dysfunction in a non-dopaminergic cell population involved in olfaction, such as the cholinergic system [29]. Interestingly, one study has shown a doubling of dopaminergic periglomular neurons in the olfactory bulb in PD [22]. As dopamine is known to inhibit transmission between axons of olfactory receptor neurons and dendrites of mitral cells in the olfactory bulb in animal models, it is possible that a tonic inhibition of olfactory neurotransmission occurs, related to a compensatory increase in dopamine receptors in extra-nigral sites [20, 27]. A PET study of long-term changes of striatal D2 receptors demonstrated evidence of a switch of early receptor upregulation to downregulation 3–5 years into the illness in patients with PD [2]. Therefore, dopamine receptor changes with advancing disease may provide an explanation for the observed variable olfactory function changes in the longitudinal study by Herting et al. [19]. The relative difference in the correlation coefficients between selective hyposmia and hippocampal DAT binding in the dopaminergic medication naive versus non-naive patients may reflect the smaller sample size in the drug-naive patient group, but relative differences in dopamine receptor status between these groups cannot be excluded.
A fMRI study of olfactory processing in patients with PD suggests that impairment of not only hippocampal but also amygdala function contributes to olfactory dysfunction in PD [38]. The amygdala is well known for its role in emotional processing. A cerebral blood flow PET study has shown that increased emotional valence of specific odors selectively increases amygdala perfusion and may reflect emotional components of odor memory [35]. We found more robust correlations between selective hyposmia and hippocampal rather than amygdala DAT activity but we did not evaluate effects of odor-induced emotions in our study. Our limited post hoc analysis did not indicate a significant relationship between scores on the depression rating scale and amygdala DAT binding.
Our findings must be examined in the light of some limitations of the present study. One limitation is that PET assessment of low binding areas, such as the orbitofrontal and prefrontal cortex, could not be performed because of the relatively high presence of non-specific radiotracer binding in these areas. Although PET assessment of intermediate binding areas, such as the hippocampus and amygdala, is associated with variability of the PET binding estimates, our data showed robust associations with the clinical olfactory variables. Furthermore, we found significant correlations between hippocampal and amygdala DAT binding in our patients.
Many previous studies have shown selective deficits of odor identification in PD. We previously reported banana, licorice and dill pickle the most sensitive for PD compared to controls in a US population [4]. This contrasts with pizza and oil of wintergreen as suggested by Hawkes & Shephard [18]; pizza, mint, and licorice were optimal in another British sample Silveira-Moriyama et al [33], both with USPIT. An Australian study using the 12-odor BSIT. found banana, licorice and dill pickle to be most selective [13]. A German study implementing the 12-odor Sniffin' Sticks test reported that licorice, followed by aniseed, pineapple, apple, turpentine, and banana, separated PD patients from controls [8]. These differences may reflect cultural differences in affinity with specific odors, differences in the patient populations studied or variations in the olfactory test methods applied. Furthermore, the use of the UPSIT as a research instrument has its own limitations as UPSIT odors do not have equal threshold values, and have multiple individual odors. Finally, these differences between studies may also reflect the more variable and perhaps dynamic neurochemical changes related to selective hyposmia in PD.
We conclude that selective hyposmia in PD is more robustly correlated with hippocampal than amygdala or striatal dopamine denervation. Hippocampal dopamine denervation may contribute to abnormal cognitive processing of olfactory information leading to the selective deficits in olfactory identification in PD.
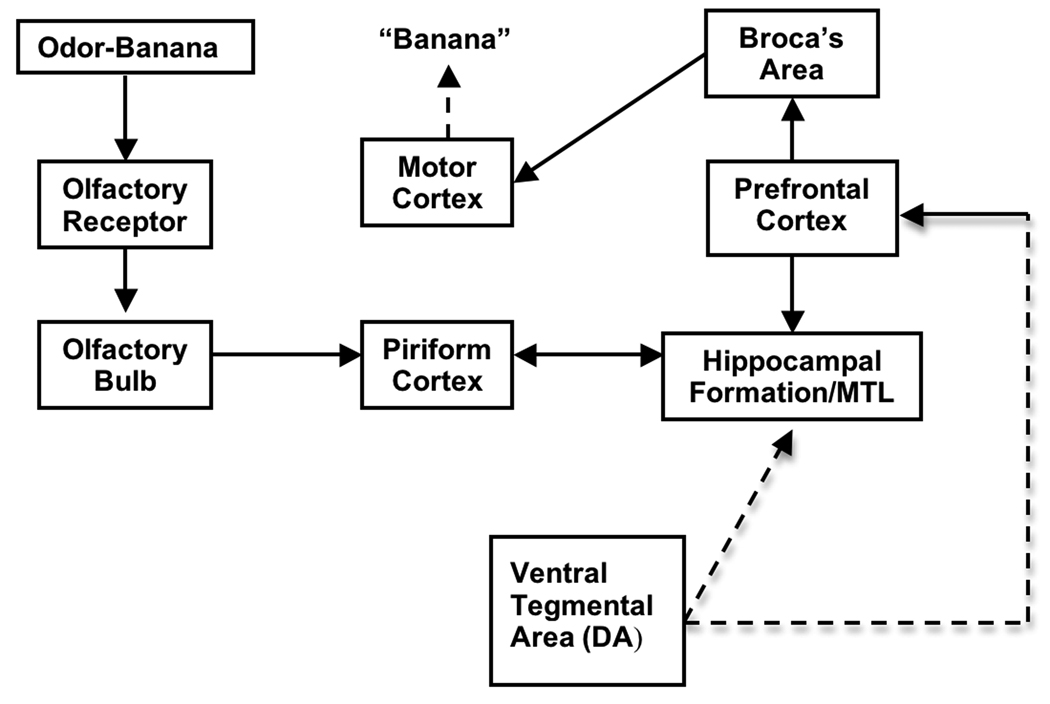
Figure 2.
Diagram showing the pathways involved in olfactory discrimination in the UPSIT. The subject is presented with an odor. This activates olfactory receptors and processing of information through the olfactory pathways. For recall of the odor name, hippocampus and prefrontal cortex are required and the verbal response requires Broca’s area and motor cortex. This process also requires mesolimbic dopamine neuron innervation of the hippocampus and prefrontal cortex, which is altered in PD (dashed lines).
Acknowledgements
The authors thank the PET technologists, cyclotron operators, chemists, Kurt Schimmel, Larry Ivanco, and study coordinators for their assistance. Supported by NIH P01 NS019608.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA. Spared caudal brainstem SERT binding in early Parkinson's disease. J Cereb Blood Flow Metab. 2008;28:441–444. doi: 10.1038/sj.jcbfm.9600599. [DOI] [PubMed] [Google Scholar]
- 2.Antonini A, Schwarz J, WH O, Pogarell O, Leenders KL. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson's disease: a study with positron emission tomography and [11C]raclopride. Mov Disord. 1997;12:33–38. doi: 10.1002/mds.870120107. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Bohnen NI, Gedela S, Kuwabara H, Constantine GM, Mathis CA, Studenski SA, Moore RY. Selective hyposmia and nigrostriatal dopaminergic denervation in Parkinson's disease. J Neurol. 2007;254:84–90. doi: 10.1007/s00415-006-0284-y. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 6.Casteels C, Lauwers E, Bormans G, Baekelandt V, Van Laere K. Metabolic-dopaminergic mapping of the 6-hydroxydopamine rat model for Parkinson's disease. Eur J Nucl Med Mol Imaging. 2008;35:124–134. doi: 10.1007/s00259-007-0558-3. [DOI] [PubMed] [Google Scholar]
- 7.Coopersmith R, Weihmuller FB, Kirstein CL, Marshall JF, Leon M. Extracellular dopamine increases in the neonatal olfactory bulb during odor preference training. Brain Res. 1991;564:149–153. doi: 10.1016/0006-8993(91)91365-8. [DOI] [PubMed] [Google Scholar]
- 8.Daum RF, Sekinger B, Kobal G, Lang CJG. Riechprüfung mit "sniffin' sticks" zur klinischen Diagnostic des Morbus Parkinson. Nervenarzt. 2000;71:643–650. doi: 10.1007/s001150050640. [DOI] [PubMed] [Google Scholar]
- 9.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 10.Doty RL, Li C, Bagla R, Huang W, Pfeiffer C, Brosvic GM, Risser JM. SKF 38393 enhances odor detection performance. Psychopharmacology (Berl) 1998;136:75–82. doi: 10.1007/s002130050541. [DOI] [PubMed] [Google Scholar]
- 11.Doty RL, Risser JM. Influence of the D-2 dopamine receptor agonist quinpirole on the odor detection performance of rats before and after spiperone administration. Psychopharmacology (Berl) 1989;98:310–315. doi: 10.1007/BF00451680. [DOI] [PubMed] [Google Scholar]
- 12.Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992;55:138–142. doi: 10.1136/jnnp.55.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Double KL, Rowe DB, Hayes M, Chan DK, Blackie J, Corbett A, Joffe R, Fung VS, Morris J, Halliday GM. Identifying the pattern of olfactory deficits in Parkinson disease using the brief smell identification test. Arch Neurol. 2003;60:545–549. doi: 10.1001/archneur.60.4.545. [DOI] [PubMed] [Google Scholar]
- 14.Dujardin K, Duhamel A, Becquet E, Grunberg C, Defebvre L, Destee A. Neuropsychological abnormalities in first degree relatives of patients with familial Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:323–328. doi: 10.1136/jnnp.67.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto Y, Grace AA. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex. 2008;18:1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkes CH. Parkinson's disease and aging: same or different process? Mov Disord. 2008;23:47–53. doi: 10.1002/mds.21766. [DOI] [PubMed] [Google Scholar]
- 17.Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkes CH, Shephard BC. Selective anosmia in Parkinson's disease? Lancet. 1993;341:435–436. [PubMed] [Google Scholar]
- 19.Herting B, Schulze S, Reichmann H, Haehner A, Hummel T. A longitudinal study of olfactory function in patients with idiopathic Parkinson's disease. J Neurol. 2008;255:367–370. doi: 10.1007/s00415-008-0665-5. [DOI] [PubMed] [Google Scholar]
- 20.Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol. 1999;82:1082–1085. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]
- 21.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathologic study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson's disease. Mov Disord. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 23.Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- 24.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- 25.Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease: a critical analysis of alpha-synuclein staging. Neuropathol Appl Neurobiol. 2008;34:284–295. doi: 10.1111/j.1365-2990.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 26.Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD. Functional anatomy of human odor sensation, discrimination, and identification in health and aging. Neuropsychology. 2003;17:482–495. doi: 10.1037/0894-4105.17.3.482. [DOI] [PubMed] [Google Scholar]
- 27.Kranick SM, Duda JE. Olfactory dysfunction in Parkinson's disease. Neurosignals. 2008;16:35–40. doi: 10.1159/000109757. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrino D, Cicchetti F, Wang X, Zhu A, Yu M, Saint-Pierre M, Brownell AL. Modulation of dopaminergic and glutamatergic brain function: PET studies on parkinsonian rats. J Nucl Med. 2007;48:1147–1153. doi: 10.2967/jnumed.106.037796. [DOI] [PubMed] [Google Scholar]
- 29.Pignatelli A, Belluzzi O. Cholinergic modulation of dopaminergic neurons in the mouse olfactory bulb. Chem Senses. 2008;33:331–338. doi: 10.1093/chemse/bjm091. [DOI] [PubMed] [Google Scholar]
- 30.Potagas C, Dellatolas G, Ziegler M, Leveteau J, Bathien N, Mac Leod P, Rondot P. Clinical assessment of olfactory dysfunction in Parkinson's disease. Mov Disord. 1998;13:394–399. doi: 10.1002/mds.870130304. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 32.Siderowf A, Newberg A, Chou KL, Lloyd M, Colcher A, Hurtig HI, Stern MB, Doty RL, Mozley PD, Wintering N, Duda JE, Weintraub D, Moberg PJ. [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology. 2005;64:1716–1720. doi: 10.1212/01.WNL.0000161874.52302.5D. [DOI] [PubMed] [Google Scholar]
- 33.Silveira□Moriyama L, Williams D, Katzenschlager R, Lees AJ. Pizza, mint, and licorice: Smell testing in Parkinson's disease in a UK population. Mov Disord. 2005;20 Suppl 10:P471. [Google Scholar]
- 34.Tillerson JL, Caudle WM, Parent JM, Gong C, Schallert T, Miller GW. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav Brain Res. 2006;172:97–105. doi: 10.1016/j.bbr.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Vermetten E, Schmahl C, Southwick SM, Bremner JD. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull. 2007;40:8–30. [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60:510–514. doi: 10.1093/gerona/60.4.510. [DOI] [PubMed] [Google Scholar]
- 37.Wei CJ, Linster C, Cleland TA. Dopamine D(2) receptor activation modulates perceived odor intensity. Behav Neurosci. 2006;120:393–400. doi: 10.1037/0735-7044.120.2.393. [DOI] [PubMed] [Google Scholar]
- 38.Westermann B, Wattendorf E, Schwerdtfeger U, Husner A, Fuhr P, Gratzl O, Hummel T, Bilecen D, Welge-Luessen A. Functional imaging of the cerebral olfactory system in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2008;79:19–24. doi: 10.1136/jnnp.2006.113860. [DOI] [PubMed] [Google Scholar]
- 39.Yue EL, Cleland TA, Pavlis M, Linster C. Opposing effects of D1 and D2 receptor activation on odor discrimination learning. Behav Neurosci. 2004;118:184–190. doi: 10.1037/0735-7044.118.1.184. [DOI] [PubMed] [Google Scholar]