Abstract
The endocannabinoid 2-arachidonoylglycerol (2-AG) has been implicated as a key retrograde mediator in the nervous system based on pharmacological studies using inhibitors of the 2-AG biosynthetic enzymes diacyglycerol lipase α and β (DAGL- α/β). Here, we show by competitive activity-based protein profiling that the DAGL- α/β inhibitors, tetrahydrolipstatin (THL) and RHC80267, block several brain serine hydrolases with potencies equal to or greater than their inhibitory activity against DAGL enzymes. Interestingly, a minimal overlap in target profiles was observed for THL and RHC80267, suggesting that pharmacological effects observed with both agents may be viewed as good initial evidence for DAGL-dependent events.
The endogenous cannabinoid (endocannabinoid) system consists of a set of G-protein coupled receptors (CB1 and CB2), natural lipid ligands [N-arachidonoyl ethanolamine (anandamide) and 2-arachidonoylglycerol (2-AG)], and enzymatic pathways for ligand biosynthesis and degradation.1 The CB1 receptor is widely distributed throughout the mammalian nervous system, raising provocative questions about how endocannabinoid signaling is regulated in specific brain regions and neural circuits. Unlike more classical neurotransmitter systems, such as the monoamine or glutamatergic systems, where receptor diversification serves as a key mechanism to vary signaling outputs, the endocannabinoid system appears to achieve this goal, at least in part, by producing multiple ligands. Indeed, the biosynthetic and degradative pathways for anandamide and 2-AG are mediated by distinct sets of enzymes and accumulating evidence suggests that these pathways are differentially regulated in the nervous system.1
Two key enzymes implicated in the biosynthesis of 2-AG are diacylglycerol lipase (DAGL)-α and β.2 DAGL-α and β are both integral membrane proteins with four predicted transmembrane domains followed by a catalytic domain that conforms to the general sequence requirements for a serine hydrolase (including the presence of the canonical GXSXG active site motif).
Multiple lines of evidence suggest that DAGL-α/β play a role in regulating 2-AG biosynthesis in neurons. For instance, overexpression of DAGL-α in the mouse neuroblastoma cell line Neuro-2a results in a significant increase in basal 2-AG levels.3 Conversely, RNA interference-mediated knockdown of DAGL-α in Neuro-2a cells reduced basal levels of 2-AG and blocked the production of this endocannabinoid stimulated by agonists of group 1 metabotropic glutamate receptors. 3
DAGL-α/β are inhibited by two small-molecule agents, RHC80267 and tetrahydrolipstatin (THL)2,4 (Figure 1), which have been used to block many of the CB1-dependent forms of neuronal plasticity observed in in vitro preparations.5 These findings have invoked 2-AG as the principle endocannabinoid involved in retrograde signaling in the nervous system. However, this conclusion is predicated on the assumption that RHC80267 and THL selectively target DAGL-α/β in the nervous system, a hypothesis that, to date, has gone largely untested. These agents likely inhibit DAGL-α/β by covalent reaction with the enzymes' serine nucleophile (forming carbamoylated and esterified products, respectively), which suggests that the compounds might target additional serine hydrolases in the nervous system via a similar mechanism. This is certainly the case in peripheral tissues, where, for example, THL has found clinical utility as an anti-obesity agent due to blockade of pancreatic lipases in the intestine.6
Figure 1.
Structures of inhibitors of the 2-AG biosynthetic enzymes DAGL-α/β.
To more globally assess the selectivity of RHC80267 and THL, we analyzed these inhibitors by competitive activity-based protein profiling (ABPP).7 ABPP is a chemical proteomic method that utilizes active site-directed small-molecule probes to assess the functional state of numerous enzymes in parallel directly in native biological systems. In competitive ABPP, inhibitors are evaluated for their ability to impair probe labeling of target enzymes.8 Because ABPP probes typically label many members from a given enzyme class, competitive profiling experiments provide an excellent assessment of both the potency and selectivity of inhibitors. Inhibitors of DAGL-α/β are most commonly used in nervous system preparations;3,5 we therefore elected to profile these agents against a mouse brain proteome using fluorophosphonate (FP) ABPP probes, which broadly target enzymes from the serine hydrolase.9 For initial comparison, we also analyzed two lipid-based FP inhibitors, O-3841 and MAFP (Figure 1), which have also been shown to inhibit DAGL-α/β.4 The mouse brain membrane proteome was treated with each inhibitor across a broad concentration range (0.01- 100 μM) for 30 min, after which reactions were incubated with a rhodamine-tagged FP probe (FP-Rh) for 60 min, separated by SDS-PAGE, and analyzed by in-gel fluorescence scanning.
O-3841 and MAFP were found to inhibit probe labeling of numerous serine hydrolase activities, including fatty acid amide hydrolase (FAAH), KIAA1363, monoacylglycerol lipase (MAGL), ABHD6, and ABHD12 (Figure 2A). Most of these enzymes with the exception of KIAA1363 were more potently inhibited by MAFP than O-3841. In contrast, RHC80267 and THL showed more selective patterns of enzyme inhibition, blocking the labeling of three and two hydrolase activities, respectively (Figure 2B). None of these enzymes correspond to DAGL-α/β, large enzymes (> 70 kDa) that appear to be poorly labeled by FP-probes.10
Figure 2.

Competitive ABPP of DAGL-α/β inhibitors with a mouse brain membrane proteome. A, Profiles for the fluorophosphonate inhibitors O-3841 and MAFP. B, Profiles for RHC80267 and THL.
To identify the targets of RHC80267 and THL, mouse brain proteome was treated with each inhibitor (50 and 5 μM, respectively) or DMSO (control) for 30 min and then incubated with a biotinylated FP-probe (FP-biotin, 5 μM) for 120 min. Probe-labeled proteins were enriched and characterized by a combination of avidin affinity and liquid chromatography-mass spectrometry steps, following previously described procedures.11 All samples were analyzed in triplicate. This approach, termed ABPP-MudPIT, identified 35 brain serine hydrolases at sufficiently high spectral counts (> 15 average counts in control proteomes) for comparative quantitation between inhibitor- and DMSO-treated proteomes (Figure 3A).
Figure 3.
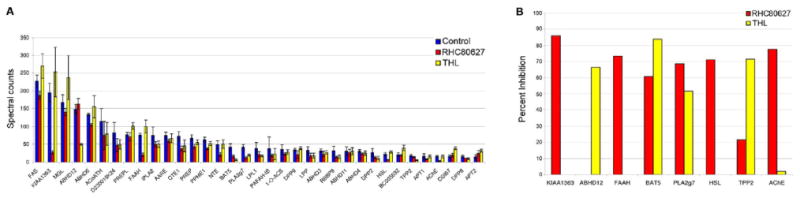
Identification of brain serine hydrolase targets of RHC80267 and THL by ABPP-MudPIT. A, Average spectral count values for brain serine hydrolases in control (DMSO-treated), RHC80267-treated (50 μM), and THL-treated (5 μM) mouse brain proteomes. Data represent average values ± standard errors for three independent experiments. B, Brain serine hydrolases that showed ≥ 60% blockade of probe labeling in RHC80267- and/or THL-treated proteomes.
Candidate targets of RHC80267 and THL were defined as serine hydrolases that showed greater than 60% reductions in average spectral counts in inhibitor-treated versus DMSO-treated proteomes. This criterion identified six and three putative targets of RHC80267 and THL, respectively (Figure 3B). These hydrolases included enzymes implicated in endocannabinoid metabolism, such as FAAH12 and ABHD12,13 enzymes that regulate other signaling molecules, such as acetylcholine [acetylcholinesterase (AChE)14], platelet-activating factor (PLA2g715) and lysophosphatidic acid (KIAA136316), as well as uncharacterized enzymes (BAT5). Comparison of these results to the gel-based profiles shown in Figure 2 led to the tentative assignment of the 60 kDa hydrolase sensitive to both RHC80267 and THL as BAT5. Additional hydrolases detected by LC-MS analysis (e.g., PLA2g7, AChE) were not visible by gel analysis, underscoring the enhanced sensitivity of the former analytical method. Neither DAGL-α nor β were detected by ABPP-MudPIT, indicating that these hydrolase are either very low abundance in mouse brain and/or weakly labeled by FP probes.10
We next set out to confirm a subset of the serine hydrolase targets of RHC80267 and THL. HEK293T cells were transfected with cDNAs encoding representative targets of RHC80267 (KIAA1363, FAAH, BAT5, PLA2g7) and THL (ABHD12, BAT5, PLA2g7), and transfected proteomes were then treated with RHC80267 and THL (0.01-100 μM), followed by FP-Rh, which permitted calculation of IC50 values for inhibition of each enzyme (Figure 4). ABHD12, BAT5, and PLA2g7 were inhibited by THL with high potencies (IC50 values < 100 nM) that rivaled the reported potency for THL inhibition of DAGL-α/β (IC50 value of 60 nM4). Targets of RHC80267 were inhibited with much lower potency (IC50 values between 10-70 μM), as has also been observed with DAGL-α/β, for which treatment with 30-100 μM of RHC80267 is typically used for inhibition.2,5
Figure 4.
Recombinant expression and characterization of serine hydrolase targets of RHC80267 and THL. Data represent average values ± standard errors for three independent experiments. Asterisk denotes lower band of double that corresponds to ABHD12 in transfected HEK293T proteome.
Our recombinant expression studies further confirmed the selectivity that RHC80267 and THL show for individual brain hydrolases. For instance, both FAAH and KIAA1363 were inhibited by RHC80267, but not THL, while ABHD12 was selectively blocked by THL. Only two hydrolases, BAT5 and PLA2g7 were inhibited by both RHC80267 and THL. These results indicate that, while RHC80267 and THL each inactivate several brain serine hydrolases, the overlap in their target profiles beyond DAGL-α/β is quite small (Figure 5). We finally confirmed that none of the newly identified targets of RHC80267 and THL exhibited significant hydrolytic activity using 1-stearoyl-2-arachidonoyl glycerol as a substrate, indicating that these enzymes are not themselves DAGLs (Figure 6). Collectively, these data lead us to conclude that pharmacological effects observed with both RHC80267 and THL may be viewed as good initial evidence for DAGL-α/β-dependent events, especially in systems where endocannabinoid pathways have been implicated to function. On the other hand, pharmacological effects observed selectively with THL or RHC80267 may reflect the inactivation of distinct enzymatic pathways and should therefore be interpreted with caution.
Figure 5.

Venn diagram showing unique and overlapping serine hydrolase targets of RHC80267 and THL.
Figure 6.

DAGL activity for recombinant hydrolases as measured using the substrate 1-stearoyl-2-arachidonoyl glycerol. Product formation (stearic acid, 2-AG) was monitored by LC-MS following general procedures described previously.13
Acknowledgments
We thank Gabriel Simon for assistance with figure composition and gratefully acknowledge the support of the NIH (DA017259, DA025285), the Helen L. Dorris Institute for the Study of Neurological and Psychiatric Disorders in Children and Adolescencts, and the Skaggs Institute for Chemical Biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Ahn K, McKinney MK, Cravatt BF. Chem Rev. 2008;108:1687. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Di Marzo V, Bisogno T, De Petrocellis L. Chemistry & Biology. 2007;14:741. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]; (c) Patricelli MP, Cravatt BF. Vitam Horm. 2001;62:95. doi: 10.1016/s0083-6729(01)62002-8. [DOI] [PubMed] [Google Scholar]; (d) Lambert DM, Fowler CJ. J Med Chem. 2005;48:5059. doi: 10.1021/jm058183t. [DOI] [PubMed] [Google Scholar]; (e) Okamoto Y, Wang J, Morishita J, Ueda N. Chem Biodivers. 2007;4:1842. doi: 10.1002/cbdv.200790155. [DOI] [PubMed] [Google Scholar]
- 2.Bisogno T, Howell F, WIlliams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. J Cell Biol. 2003;163:463. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. Mol Pharmacol. 2007;72:612. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- 4.Bisogno T, Cascio MG, Saha B, Mahadevan A, Urbani P, Minassi A, Appendino G, Saturnino C, Martin B, Razdan R, Di Marzo V. Biochim Biophys Acta. 2006;1761:205. doi: 10.1016/j.bbalip.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.(a) Chevaleyre V, Takahashi KA, Castillo PE. Annu Rev Neurosci. 2006;29:37. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]; (b) Hashimotodani Y, Ohno-Shosaku T, Watanabe M, Kano M. J Physiol. 2007;584:373. doi: 10.1113/jphysiol.2007.137497. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. J Neurosci. 2007;27:3663. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Safo PK, Regehr WG. Neuron. 2005;48:647. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]; (e) Straiker A, Mackie K. J Physiol. 2005;569:501. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Baer WU, Freiman I. J Physiol. 2006;577:263. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Edwards DA, Kim J, Alger BE. J Neurophysiol. 2006;95:67–75. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]; (h) Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Neuropharmacology. 2008;54:58. doi: 10.1016/j.neuropharm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Henness S, Perry CM. Drugs. 2006;66:1625. doi: 10.2165/00003495-200666120-00012. [DOI] [PubMed] [Google Scholar]
- 7.(a) Cravatt BF, Wright AT, Kozarich JW. Annu Rev Biochem. 2008;77:383. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]; (b) Jessani N, Cravatt BF. Curr Opin Chem Biol. 2004;8:54. doi: 10.1016/j.cbpa.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 8.(a) Leung D, Hardouin C, Boger DL, Cravatt BF. Nat Biotechnol. 2003;21:687. doi: 10.1038/nbt826. [DOI] [PubMed] [Google Scholar]; (b) Kidd D, Liu Y, Cravatt BF. Biochemistry. 2001;40:40005. doi: 10.1021/bi002579j. [DOI] [PubMed] [Google Scholar]; (c) Greenbaum DC, Arnold WD, Lu F, Hayrapetian L, Baruch A, Krumrine J, Toba S, Chehade K, Bromme D, Kuntz ID, Bogyo M. Chem Biol. 2002;9:1085. doi: 10.1016/s1074-5521(02)00238-7. [DOI] [PubMed] [Google Scholar]; (d) Alexander JP, Cravatt BF. Chem Biol. 2005;12:1179. doi: 10.1016/j.chembiol.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Li W, Blankman JL, Cravatt BF. J Amer Chem Soc. 2007;129:9594. doi: 10.1021/ja073650c. [DOI] [PubMed] [Google Scholar]
- 9.(a) Liu Y, Patricelli MP, Cravatt BF. Proc Natl Acad Sci USA. 1999;96:14694. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Patricelli MP, Giang DK, Stamp LM, Burbaum JJ. Proteomics. 2001;1:1067. doi: 10.1002/1615-9861(200109)1:9<1067::AID-PROT1067>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.We recombinantly expressed both DAGL-α and β in COS-7 cells, which resulted in detectable levels of protein by western blotting and activity using a substrate assay. However, neither enzyme showed strong labeling with FP-Rh, suggesting that they react poorly with this chemical probe.
- 11.Jessani N, Niessen S, Wei BQ, Nicolau M, Humphrey M, Ji Y, Han W, Noh DY, Yates JR, 3rd, Jeffrey SS, Cravatt BF. Nat Methods. 2005;2:691. doi: 10.1038/nmeth778. [DOI] [PubMed] [Google Scholar]
- 12.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Nature. 1996;384:83. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 13.Blankman JL, Simon GM, Cravatt BF. Chem Biol. 2007;14:1347. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman G, Soreq H. Cell Tissue Res. 2006;326:655. doi: 10.1007/s00441-006-0239-8. [DOI] [PubMed] [Google Scholar]
- 15.Tjoelker LW, Wilder C, Eberhardt C, Stafforini DM, Dietsch G, Schimpf B, Hooper S, le Trong H, Cousens LS, Zimmerman GA, Yamada Y, McIntyre TM, Prescott SM, Gray PW. Nature. 1995;374:549. doi: 10.1038/374549a0. [DOI] [PubMed] [Google Scholar]
- 16.Chiang KP, Niessen S, Saghatelian A, Cravatt BF. Chem Biol. 2006;13:1041. doi: 10.1016/j.chembiol.2006.08.008. [DOI] [PubMed] [Google Scholar]




