Abstract
Prostaglandin F2α (PGF2α) is a potent paracrine inhibitor of adipocyte differentiation. Here we show that treatment of differentiating 3T3-L1 preadipocytes with PGF2α induces the expression of DEC1, a transcriptional repressor that has previously been implicated in the inhibition of adipogenesis in response to hypoxia as a downstream effector of the hypoxia-inducible factor-1 (HIF-1) transcription factor. Surprisingly, despite performing our experiments under normal ambient oxygen conditions, we find that treatment of differentiating 3T3-L1 preadipocytes with PGF2α also results in the marked activation of HIF-1, as measured by an increase in the accumulation of the HIF-1α regulatory subunit. However, unlike the effects of hypoxia, this PGF2α-induced normoxic increase in HIF-1α is not mediated by an increase in the stability of the HIF-1α polypeptide, rather we find that PGF2α selectively increases the expression of the alternatively spliced HIF-1α I.1 mRNA isoform. Significantly, we demonstrate that the shRNA-mediated knockdown of endogenous HIF-1α expression attenuates the PGF2α-induced expression of DEC1, overcomes the inhibitory effects of PGF2α on the expression of proadipogenic transcription factors C/EBPα and PPARγ and partially rescues the PGF2α-induced inhibition of adipogenesis. Taken together, these results indicate that PGF2α promotes the activation of the HIF-1 transcription factor pathway under normal oxygen conditions, and highlight a potential role for the normoxic activation of the HIF-1/DEC1-pathway in mediating the inhibitory effects of PGF2α on adipocyte differentiation.
Keywords: PGF2α, 3T3-L1 preadipocytes, adipocyte differentiation, HIF-1, DEC1, normoxia
Adipocytes are specialized cells that play a critical role in the regulation of whole body energy homeostasis [Spiegelman and Flier, 2001]. They store energy in the form of triglycerides when food is plentiful, and conversely release energy in the form of free fatty acids during conditions of starvation. In addition to their role in energy storage, adipocytes are also known to fulfill a critical endocrine function via the secretion of factors that regulate such systemic physiological responses as food intake, insulin responsiveness, immunity and blood pressure [Fruhbeck et al., 2001; Kershaw and Flier, 2004]. Because of these important physiological roles and their known contribution to the development of obesity and its ensuing disease sequelae, understanding the mechanisms that underlie the differentiation and function of adipocytes has become an area of intense investigation.
Much of our knowledge regarding adipocyte differentiation comes from the use of in vitro cell culture model systems such as the murine 3T3-L1 preadipocyte cell line [Green and Kehinde, 1975]. These cells are committed to the adipocyte lineage and can be readily induced to undergo differentiation in culture following treatment of confluent growth-arrested cells with an adipogenic mixture comprised of methylisobutylxanthine, dexamesthasone and insulin, collectively known as MDI. Exposure of 3T3-L1 cells to this array of hormonal inducers leads to the complex interplay of a number of signal transduction pathways that set in motion a highly orchestrated cascade of sequential transcriptional events that ultimately results in the specification of the mature adipocyte cell phenotype [Otto et al., 2005; Rosen and MacDougald, 2006]. Although many transcription factors are known to play a role in the regulation of adipocyte differentiation, the most important and well characterized are the nuclear hormone receptor peroxisome proliferators-activated receptor γ (PPARγ) and members of the CCAAT/enhancer-binding protein (C/EBP) family [Otto et al., 2005; Rosen and MacDougald, 2006]. C/EBPβ and C/EBPδ are amongst the first transcription factors induced during the initial stages of adipocyte differentiation in response to hormonal stimulation [Otto et al., 2005; Rosen and MacDougald, 2006]. These two early transcription factors are responsible for helping to directly promote the expression of the late proadipogenic transcription factors PPARγ and C/EBPα [Yeh et al., 1995; Wu et al., 1996]. Once expressed, PPARγ and C/EBPα act in an autoregulatory loop to re-enforce each other's expression, then act coordinately to promote the expression of a panel of genes responsible for the establishment of the terminally differentiated adipocyte phenotype [Otto et al., 2005; Rosen and MacDougald, 2006].
A wealth of data indicates that the efficiency of adipocyte differentiation can be influenced by the presence of a variety of paracrine factors such as growth factors, cytokines and other intercellular signaling molecules that act to either enhance, or inhibit, the extent of the adipogenic process [MacDougald and Mandrup, 2002]. One of the paracrine factors known to potently inhibit adipocyte differentiation is the prostaglandin molecule PGF2α [Serrero et al., 1992; Lepak and Serrero, 1993; Casimir et al., 1996]. Recent work from our laboratory has shown that PGF2α inhibits adipocyte differentiation by specifically blocking the expression of the proadipogenic transcription factors PPARγ and C/EBPα [Liu and Clipstone, 2007]. Intriguingly, the inhibitory effects of PGF2α on the expression of C/EBPα and PPARγ and ensuing adipocyte differentiation, are reversed in the presence of trichostatin A, a specific inhibitor of histone deacetylase (HDAC) enzymes [Liu and Clipstone, 2007]. Since HDACs are known to be involved in mediating the inhibitory effects of transcriptional repressors on gene transcriptional events [Ng and Bird, 2000] this has led us to propose a model in which PGF2α inhibits adipogenesis via the action of a transcriptional repressor that is capable of directly inhibiting the expression of PPARγ and C/EBPα.
A number of transcriptional repressors are known to be expressed in 3T3-L1 preadipocytes [Tong et al., 2000; Yun et al., 2002; Banerjee et al., 2003; Shi et al., 2003; Davis et al., 2004; Armoni et al., 2006; Ross et al., 2006]. Amongst these genes, the basic helix-loop-helix transcription factor DEC1 (also known as Stra13, Sharp2, and Bhlhb2) is known to associate with HDACs [Sun and Taneja, 2000] and has been shown to potently inhibit 3T3-L1 preadipocyte differentiation by preventing the expression of the proadipogenic transcription factor PPARγ [Yun et al., 2002]. DEC1 is implicated in mediating the inhibitory effects of hypoxia on adipocyte differentiation, its expression is transcriptionally induced in hypoxia-exposed 3T3-L1 preadipocytes via the actions of the HIF-1 transcription factor, and it is believed to inhibit adipogenesis by directly repressing the expression of the PPARγ2 promoter [Yun et al., 2002].
HIF-1 is the principal transcription factor involved in the transcriptional response to hypoxia and is responsible for the hypoxia-induced expression of DEC1 in 3T3-L1 preadipocytes [Yun et al., 2002], it is a member of the basic helix-loop-helix Per, Arnt and Sim family and is comprised of two subunits: a HIF-1α regulatory subunit and a constitutively expressed HIF-1β subunit [Wang et al., 1995]. Under normal oxygen conditions the HIF-1α polypeptide is highly unstable due to its post-translational modification by oxygen-dependent prolyl hydroxylase enzymes that promote the binding of the von Hippel Landau E3 ubiquitin ligase, leading to HIF-1α polyubiquitination and its subsequent targeting to the proteosome for degradation [Bruick, 2003]. Conversely, under hypoxic conditions, the HIF-1α polypep-tide no longer undergoes proline hydroxylation and hence its expression is stabilized, allowing it to translocate into the nucleus where it dimerizes with HIF-1β and binds to its cognate sites within the promoters of HIF-1 target genes [Bruick, 2003]. While this hypoxia-induced HIF-1α protein stabilization is certainly the best known mechanism of HIF-1α regulation, it has become increasingly apparent that there are additional signaling pathways and mechanisms that allow for the activation of HIF-1 under normal oxygen conditions [Laughner et al., 2001; Lukashev et al., 2001; Chan et al., 2002; Page et al., 2002; Jung et al., 2003; Hirota et al., 2004]. However, the physiological significance of HIF-1 activated under such normoxic conditions is currently not well understood.
In the current study we have extended our investigations into the molecular mechanisms underlying the inhibitory effects of PGF2α on adipocyte differentiation. Surprisingly, despite the use of normal oxygen conditions for our experiments, we find that treatment of differentiating 3T3-L1 preadipocytes with PGF2α results in the marked activation of the HIF-1 transcription factor and the ensuing expression of the DEC1 transcriptional repressor. Moreover, we provide evidence that this normoxic activation of HIF-1 likely plays a role in mediating the inhibitory effects of PGF2α on adipocyte differentiation.
MATERIALS AND METHODS
Cell Culture and Adipocyte Differentiation
3T3-L1 preadipocytes (ATCC) were cultured in Dulbecco's modified Eagle's medium containing high glucose (Invitrogen) supplemented with 10% (v/v) fetal calf serum (Hyclone), 100 U/ml penicillin G (Invitrogen), and 100 μg/ml streptomycin (Invitrogen), and induced to undergo differentiation essentially as previously described [Neal and Clipstone, 2002]. In brief, 5 × 104 cells were plated/well of a 6-well plate and allowed to reach confluence. Two-days post-confluence (day 0) cells were treated for 2 days in growth media plus MDI (0.5 mM Methylisobutylxanthine, 1 μM Dexamethasone, and 10 μg/ml Insulin; all from Sigma). The cells were re-fed with growth media containing 10 μg/ml insulin at day 2 and every 2 days thereafter with growth media alone. Where indicated, cells were additionally treated for the first 48 h period of the differentiation process with either PGF2α (100 ng/ml), or CoCl2 (100 μM), these concentrations were chosen based upon the literature and preliminary dose response experiments. The extent of cellular differentiation was assessed following fixation with formalin and staining with the lipophilic dye Oil Red O (Sigma).
Plasmid Constructs
The retrovirus expressing constitutively active HIF-1α (MSCV-caHIF-1α) was generated by inserting a 2.5 kb Bam HI fragment encoding a constitutively active murine HIF-1α mutant containing alanine substitutions at critical regulatory proline residues (a kind gift of Dr. Mary Hunzicker-Dunn) into the Bgl II site of MSCV-GFP. For the HIF-1α shRNA construct, oligonucleotides corresponding to a previously published functional HIF-1α shRNA [Aminova et al., 2005] (SHHIF404A: 5'-GATCCCCTGTGAGCTCACATCTT GATTTCAAGAGAATCAAGATGTGAGCTCACATTTTTGGAAA-3' and SHHIF404B: 5'-AGCTTTTCCAAAAATGTGAGCTCACATCTTGATTCTCTTGAAA TCAAGATGTGAGCTCACAGGG-3') were synthesized, annealed and cloned into the Bgl II and HindIII sites of the pSUPER-RETRO retroviral vector (OligoEngine) immediately downstream of the murine H1 promoter. The MSCV-ODD-luciferase reporter vector containing the oxygen-dependent degradation (ODD) domain of human HIF-1α fused in-frame with firefly luciferase was generated as follows. A DNA fragment corresponding to amino acid residues 530-603 of human HIF-1α was PCR amplified from human cDNA using the forward primer 5'-CCGCTCGAGACCATGGAATTCAAGTTGGAATTGGTAG-3' and 5'-GAGCCATGGCCTGGAATACTGTAACTGTGCTTTGAG-3' as a reverse primer. The forward primer introduced a Xho I cloning site and a Kozak consensus initiator methionine, while the reverse primer introduced an Nco I site that is in frame with the Nco I site located over the initiator methionine in the firefly luciferase gene contained in pGL3-Luciferase (Promega). The PCR fragment was then digested with Xho I and Nco I and inserted into Xho I/Nco I digested pGL3-Luciferase, and the integrity of the sequence was confirmed by DNA sequencing. The resulting plasmid was digested with Xba I, blunted, then digested with Xho I and the resulting ODD-Luciferase fragment was cloned into the MSCV-GFP retroviral expression plasmid at the Xho I and Hpa I sites.
Retroviral Production and Infection of 3T3-L1 Preadipocytes
Retroviral expression vectors were co-transfected together with pVSV-G (Clontech) into the GP293 pantropic packaging cell line (Clontech) using Lipofectamine Plus (Invitrogen) as per the manufacturer's instructions. Media was replaced after 24 h and viral supernatants were harvested 2 days post-transfection and stored at -80°C. For infections, 5 × 104 3T3-L1 cells were plated per well of a 6-well plate. The next day, media was replaced with 2 ml of viral supernatant containing 8 μg/ml polybrene (Sigma) and cells were spin-infected [Pear, 2003] by centrifugation at 2,000 rpm for 1.5 h at room temperature. After removal of viral supernatant, normal growth media was added and cells were expanded for subsequent analysis.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from 3T3-L1 cells by RNeasy kit (QIAGEN, Valencia, CA) at the indicated times following induction of differentiation and cDNA was synthesized with an oligo-dT primer and reverse transcriptase (Promega). PCR amplification was performed using gene-specific primers for DEC1 (forward primer, 5'-agagacgtgac cggattac-3', reverse primer, 5'-cggtatcttgtctgggttca-3'); HIF-1α I.1 exon (forward primer, 5'-tttctgggcaaactgtta-3', reverse primer, 5'-taaccc catgtatttgttc-3') and HIF-1α 1.2 exon (forward primer, 5'-CGCCTCTGGACTT GTCTCTTTC-3', reverse primer, 5'-taaccccatgtatttgttc-3'). HPRT (forward primer, 5'-GTTGGATACAGGCCAGACTTTGTTG-3'; reverse primer, 5'-GAGGGTA GGCTGGCCTATAGGCT-3') served as a loading control. PCR products were resolved by 1.5% agarose gel electrophoresis, visualized with ethidium bromide and photographed.
Luciferase Assay
3T3-L1 cells infected with the ODD-Luciferase virus were induced to undergo differentiation using the standard MDI protocol in the additional presence where indicated of either vehicle, PGF2α (100 ng/ml), or CoCl2 (100 μM). After 2 days, cell lysates were prepared from triplicate cultures and firefly luciferase assays were performed using the Luciferase Assay System kit (Promega) according to the manufacturer's protocol. Luciferase activity was normalized by the total amount of cellular protein as determined by the Quant-iT™ protein assay kit (Invitrogen).
Immunoblot Analysis
At the indicated times following induction of differentiation, 3T3-L1 cells were lysed in RIPA buffer (0.1% SDS, 1% deoxycholate, 1 mM EDTA, 1% TX-100, 50 mM Tris, pH 8.0, 500 mM NaCl), centrifuged at 100,000 rpm for 20 min at 4°C to pellet chromatin (Beckman TL-100 ultra-centrifuge) and the supernatant taken for further analysis. For the analysis of HIF-1α polypeptide levels nuclear extracts were prepared using a nuclear extraction kit (Pierce) according to the manufacturer's instructions. In experiments to assess the stability of the HIF-1α polypeptide, the protein synthesis inhibitor cyclohexamide (100 μM; Sigma) was added to the media and nuclear extracts were prepared at 15 min time intervals thereafter. Protein extracts were resolved by SDS-PAGE, transferred to nitrocellulose, and subjected to immunoblot analysis with the relevant primary antibodies: PPARγ (H-100), C/EBPα (14AA), Sp1 (1C6) all purchased from Santa Cruz Biotechnology, aP2 (10004944; Cayman Chemical), anti-actin (A2668; Sigma), and anti-HIF-1α (AB1536; R&D Systems). Appropriate horseradish peroxidase-conjugated secondary antibodies (anti-rabbit and anti-mouse) were purchased from Amersham Biosciences, and detected by enhanced chemiluminescence using ECL reagents (Amersham Biosciences).
RESULTS
Treatment of Differentiating 3T3-L1 Preadipocytes With PGF2α Induces the Expression of the DEC1 Transcriptional Repressor
Our previous studies had led us to propose a model in which PGF2α might act to inhibit adipocyte differentiation by increasing the expression of an HDAC-dependent transcriptional repressor capable of directly inhibiting the expression of the C/EBPα and PPARγ proadipogenic transcription factors [Liu and Clipstone, 2007]. We therefore initiated a screen for PGF2α-induced, HDAC-associated transcriptional repressors implicated in the regulation of adipogenesis. Towards this end we analyzed the effects of PGF2α treatment on the expression of DEC1, an HDAC-associated transcriptional repressor previously implicated in inhibiting adipocyte differentiation in response to hypoxia by directly inhibiting the activity of the PPARγ2 promoter [Yun et al., 2002]. As shown in Figure 1, we found that DEC1 was not significantly expressed in growth-arrested 3T3-L1 preadipocytes induced to undergo adipocyte differentiation using the standard MDI-stimulated protocol. In contrast, however, we found that in the presence of PGF2α, DEC1 mRNA levels were markedly increased, remaining elevated through the first 3 days post-MDI stimulation, before returning to background levels by day 4.
Fig. 1.
Effects of PGF2α-treatment on the expression of DEC1 during the early stages of 3T3-L1 preadipocyte differentiation. 3T3-L1 preadipocytes were induced to undergo adipocyte differentiation by the standard MDI protocol in the presence of either vehicle or 100 nM PGF2α for the first 48 h of the culture period. Total RNA was isolated at the indicated number of days post-differentiation and the expression of DEC1 was analyzed by RT-PCR analysis. HPRT expression was determined as a control. Data are representative of three independent experiments.
PGF2α Treatment Induces the Expression of the HIF-1α Polypeptide During Adipocyte Differentiation
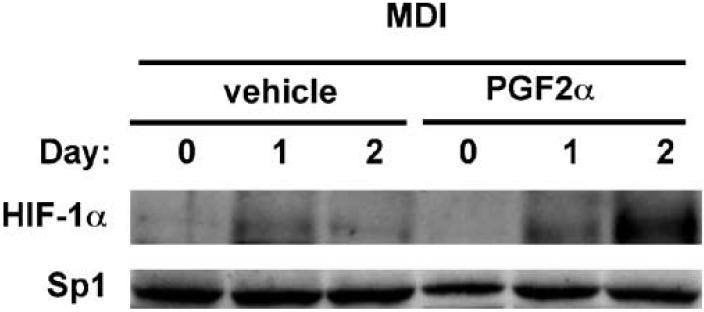
Since previous studies had demonstrated that DEC1 is transcriptionally induced in differentiating 3T3-L1 preadipocytes via the actions of the HIF-1 transcription factor [Ivanova et al., 2001; Yun et al., 2002], we next considered the possibility that PGF2α might also influence DEC1 expression via an effect on HIF-1. As the activity of HIF-1 is primarily determined by the accumulation of the HIF-1α protein subunit, we examined whether PGF2α was able to influence HIF-1α protein levels during adipocyte differentiation. Thus, 3T3-L1 cells were subjected to the classical MDI-induced differentiation protocol in the presence of either vehicle or PGF2α, and nuclear extracts were collected at daily intervals for the analysis of HIF-1α protein levels by immunoblotting. As shown in Figure 2, stimulation of 3T3-L1 cells with MDI alone resulted in a low level transient increase in the HIF-1α protein level at day 1 post-stimulation, which was reduced to essentially background levels by day 2. In contrast, we found that cells stimulated to undergo MDI-induced differentiation in the presence of PGF2α exhibited a marked elevation in HIF-1α protein levels at day 2 post-treatment. Hence, these results indicate that PGF2α acts to increase HIF-1α protein levels in differentiating 3T3-L1 preadipocytes under normal oxygen conditions.
Fig. 2.
Effects of PGF2α-treatment on HIF-1α protein levels in MDI-stimulated 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were induced to undergo adipocyte differentiation by the standard MDI protocol in the presence of either vehicle or 100 nM PGF2α. Nuclear extracts were prepared at the indicated days post-treatment and HIF-1α protein levels were determined by immunoblotting. Sample integrity was confirmed by immunoblotting for the Sp1 transcription factor as a control. Data are representative of three independent experiments.
PGF2α Treatment Does Not Promote the Increased Stability of the HIF-1α Polypeptide Subunit
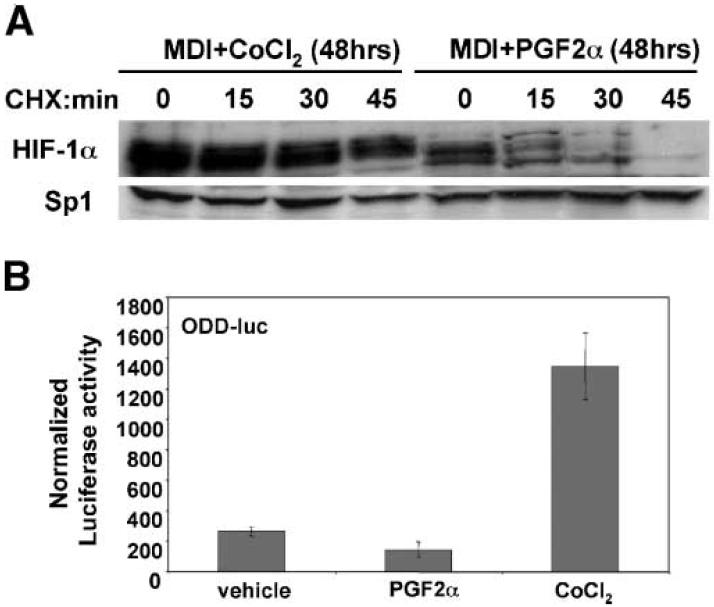
One of the major mechanisms involved in the control of HIF-1α expression is the regulation of HIF-1α protein turnover in response to changing oxygen concentrations [Bruick, 2003; Huang and Bunn, 2003]. Under normal oxygen conditions the HIF-1α protein is usually unstable and exhibits a short half-life due to the action of proline hydroxylases, which modify specific proline residues in the HIF-1α oxygen-dependent degradation (ODD) domain, that target HIF-1α for ubiquitination and degradation via the proteosome [Bruick, 2003; Huang and Bunn, 2003]. In the presence of hypoxia this pathway is blocked resulting in the stabilization of the HIF-1α polypeptide. Accordingly, in order to determine whether PGF2α affects HIF-1α levels at the level of protein stability, we first directly examined the effects of the PGF2α-treatment on the intrinsic stability of the endogenous HIF-1α polypeptide. For this experiment, 3T3-L1 cells were induced to differentiate with MDI in the presence of PGF2α for 2 days to allow time for expression of HIF-1α, then cells were treated with the protein synthesis inhibitor cyclohexamide to prevent ongoing de novo protein synthesis and nuclear extracts were collected at 15 min intervals for determination of HIF-1α protein levels by immunoblot analysis. As a control, parallel cultures were induced to differentiate in the presence of the hypoxic mimetic CoCl2 and were similarly treated with cycloheximide at 2 days post-MDI-induced differentiation. As shown in Figure 3A, in cells stimulated with MDI plus CoCl2, cyclohexamide treatment did not significantly decrease the HIF-1α protein levels throughout the 45 min time course, confirming that CoCl2 acts to prevent the turnover of the HIF-1α polypeptide. In marked contrast, however, we found that in cells stimulated with MDI plus PGF2α, cyclohexamide treatment caused HIF-1α levels to rapidly fall to essentially background levels within 45 min, due presumably to the inherent rapid turnover of the HIF-1α polypeptide under normoxic conditions.
Fig. 3.
Effects of PGF2α-treatment on HIF-1α protein stability in MDI-treated 3T3-L1 preadipocytes. A: Immunoblot analysis showing that PGF2α-treatment does not promote the stabilization of the HIF-1α polypeptide in MDI-treated 3T3-L1 preadipocyte cells. 3T3-L1 preadipocytes were induced to undergo adipocyte differentiation by the standard MDI protocol in the presence of either 100 μM CoCl2 or 100 nM PGF2a. After 48 h 100 μM cyclohexamide (CHX) was added to inhibit ongoing protein synthesis and nuclear extracts were prepared at the indicated time points and analyzed for the presence of HIF-1α by immunoblotting. The expression of Sp1 was determined as a control. B: 3T3-L1 preadipocytes were transduced with a retrovirus encoding the ODD-luciferase reporter and induced to undergo differentiation by the standard MDI protocol in the presence of either vehicle, 100 nM PGF2α, or 100 μM CoCl2. After 48 h cell lysates were prepared and analyzed for luciferase activity. Normalized luciferase levels are presented as the mean of triplicate determinations with the standard deviations indicated. Data are representative of at least two independent experiments.
As an independent means to confirm that PGF2α does not influence HIF-1α expression by affecting its intrinsic protein stability, we took advantage of a previously validated reporter gene in which the ODD domain of HIF-1α is fused in-frame with firefly luciferase, and is therefore highly responsive to intracellular signals that act to stabilize the endogenous HIF-1α polypeptide [D'Angelo et al., 2003]. Thus, 3T3-L1 preadipocytes were transduced with a recombinant retrovirus expressing the ODD-luciferase reporter and were subjected to the MDI-induced differentiation protocol in the presence of either vehicle or PGF2α. At 2 days post-stimulation, a time point at which we know that HIF-1α levels are increased in PGF2α-treated cells, but not control cells (see Fig. 2), cell extracts were prepared and were assayed for luciferase activity. As a positive control, parallel cultures were also stimulated with the hypoxic mimetic CoCl2, which is known to promote the stabilization of HIF-1α. As expected, treatment of cells with CoCl2 resulted in a significant increase in luciferase activity (Fig. 3B) due to the known ability of CoCl2 to act via the ODD domain to promote the stabilization of the HIF-1α polypeptide. In contrast, however, treatment with PGF2α did not increase the activity of the ODD-luciferase reporter relative to vehicle control (Fig. 3B), thereby indicating that PGF2α treatment does not appear to affect the intrinsic stability of the HIF-1α ODD domain. Taken together, therefore, these combined results strongly suggest that the ability of PGF2α to increase HIF-1α expression in MDI-treated 3T3-L1 preadipocytes does not involve a post-translational effect on the stability of the HIF-1α polypeptide, but rather is likely to be dependent upon ongoing de novo HIF-1α protein synthesis.
PGF2α Treatment Induces the Expression of the HIF-1α I.1 Exon-Specific mRNA Isoform During MDI-Induced Adipocyte Differentiation
Since the above data indicated that PGF2α treatment did not act to increase HIF-1α protein stability, we next considered other potential mechanisms by which PGF2α might affect HIF-1α expression levels. A number of previous studies have demonstrated that a variety of extracellular signals under normoxic conditions can increase HIF-1α protein levels by influencing HIF-1α mRNA levels [Lukashev et al., 2001; Page et al., 2002; Blouin et al., 2004]. Hence, we next evaluated whether PGF2α-treatment might affect the expression of HIF-1α mRNA. In this respect, the murine HIF-1α gene has been shown to contain two alternative first exons, I.1 and I.2, that are each generated by distinct promoters that give rise to two alternatively-spliced HIF-1α mRNA isoforms [Wenger et al., 1997, 1998]. The I.1 exon-containing mRNA has a tissue-specific and cell activation-dependent expression, whereas the I.2 exon-containing mRNA is believed to be constitutively expressed in most cell types [Wenger et al., 1997, 1998]. As shown in Figure 4, the HIF-1α I.2 exon-specific mRNA isoform is expressed in unstimulated 3T3-L1 preadipocytes at day 0, and although its expression is transiently increased following MDI-induced stimulation, its expression is not significantly affected by exposure to PGF2α. Conversely, the HIF-1α I.1 exon-specific mRNA isoform is not detectable in unstimulated cells at day 0, but is transiently induced by treatment with MDI at day 1 post-stimulation falling to background levels by day 2. Significantly, however, treatment of 3T3-L1 cells with MDI plus PGF2α results in the increased and sustained expression of this HIF-1α mRNA isoform throughout the first 3 days of the differentiation process. These data therefore indicate that PGF2α acts to selectively induce the sustained expression of the HIF-1α I.1 mRNA isoform during adipocyte differentiation, which might potentially help explain the effects of PGF2α on the increased accumulation of the HIF-1α polypeptide.
Fig. 4.
Effects of PGF2α-treatment on the expression of HIF-1α mRNA isoforms in MDI-treated 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were induced to undergo adipocyte differentiation by the standard MDI protocol in the presence of either vehicle or 100 nM PGF2α for the first 48 h of the culture period. Total RNA was isolated at the indicated number of days post-differentiation and the expression of either the HIF-1α I.1 or HIF-1a I.2 mRNA isoforms, or HPRT was analyzed by RT-PCR analysis. Data are representative of three independent experiments.
Activation of HIF-1α Is Sufficient to Inhibit Adipocyte Differentiation Under Normoxic Conditions
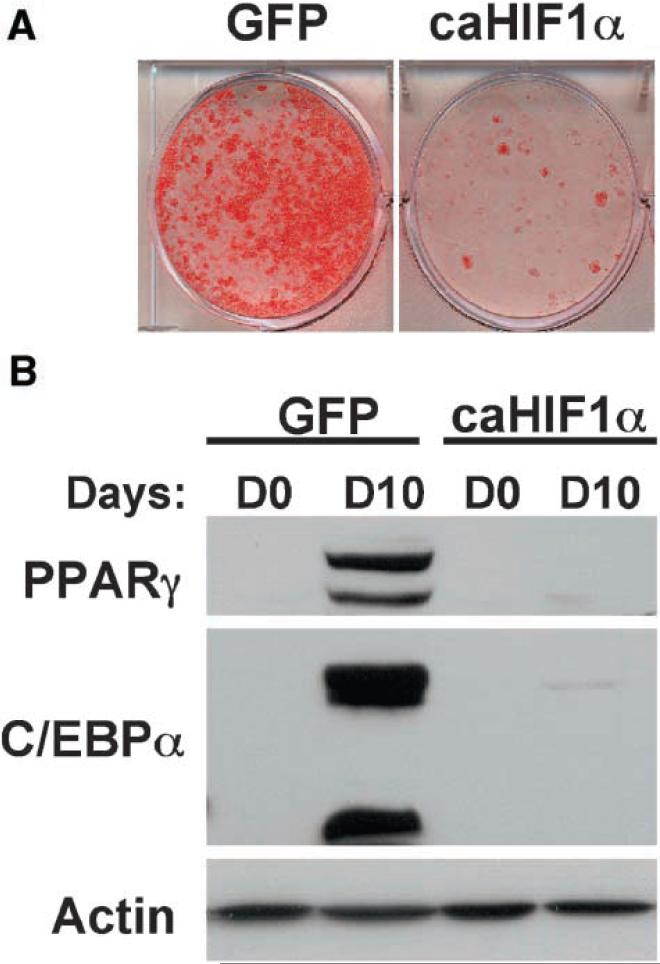
Having demonstrated that PGF2α-treatment is able to induce the normoxic activation of HIF-1 in differentiating 3T3-L1 preadipocytes, and knowing that hypoxic activation of HIF-1 has been shown to exert a potent negative influence on adipogenesis [Yun et al., 2002], we next wanted to evaluate the potential role of the normoxic activation of HIF-1 on adipocyte differentiation. To assist us in these studies, we took advantage of a constitutively active HIF-1α mutant (caHIF-1α) that is stable under conditions of normoxia and is able to activate HIF-1 dependent gene transcription in the absence of hypoxic stimulation. Hence, 3T3-L1 preadipocytes were transduced with either a control virus or with a retrovirus encoding this caHIF-1α mutant, then induced to undergo adipocyte differentiation by the standard MDI-induced protocol. As shown in Figure 5, ectopic expression of caHIF-1α was sufficient to potently inhibit MDI-induced 3T3-L1 preadipocyte differentiation, as determined by a lack of both Oil Red O staining (Fig. 5A) and failure to express the adipogenic transcription factors PPARγ and C/EBPα (Fig. 5B). Thus, these results demonstrate that normoxic activation of HIF-1α in 3T3-L1 preadipocytes is sufficient to inhibit adipocyte differentiation.
Fig. 5.
Effects of a constitutively active HIF-1α mutant on 3T3-L1 preadipocyte differentiation. A: 3T3-L1 preadipocytes transduced with either a GFP control retrovirus, or a retrovirus encoding caHIF-1α were induced to undergo adipocyte differentiation by the standard MDI protocol and stained with Oil Red O to determine the extent of differentiation at day 10. B: Whole cell extracts prepared from either GFP control- or caHIF-1α-virus transduced 3T3-L1 preadipocytes at either day 0 or day 10 post-MDI treatment were analyzed by immunoblotting for expression of PPARγ and C/EBPα. The expression of actin was determined as a control. Data are representative of at least two independent experiments.
shRNA-Mediated Knockdown of HIF-1α Partially Restores the Ability of PGF2α-Treated 3T3-L1 Preadipocytes to Undergo Adipocyte Differentiation
Finally, we wanted to investigate the potential role of the PGF2α-induced normoxic activation of HIF-1 in mediating the inhibitory effects of PGF2α on adipocyte differentiation. To accomplish this goal we took advantage of a previously characterized HIF-1α shRNA [Aminova et al., 2005] to specifically knockdown expression of endogenous HIF-1α and determine the effects on the ability of PGF2α to inhibit adipocyte differentiation. As shown in Figure 6A, retroviral-mediated expression of this HIF-1α-specific shRNA is able to successfully reduce the increased expression of HIF-1α in PGF2α-treated 3T3-L1 cells at day 2 of MDI-induced differentiation. Co-incident with this effect, we found that the HIF-1α-specific shRNA also attenuated the ability of PGF2α to induce expression of DEC1 (Fig. 6B), indicating that the PGF2α-induced expression of the DEC1 transcriptional repressor is dependent on HIF-1. Intriguingly, during initial experiments using our usual inhibitory concentration of PGF2α (100 nM), we found that while control virus-transduced cells were completely inhibited from undergoing MDI-induced differentiation, we were able to detect numerous small clusters of morphologically distinct, lipid-ladened mature adipocytes in cultures transduced with the HIF-1α shRNA virus (Fig. 6C). However, while expression of the HIF-1α shRNA consistently led to the appearance of these small clusters of morphologically differentiated adipocytes at the microscopic level, the number of these clusters was not sufficient to allow robust detection by Oil Red O staining at the macroscopic level (data not shown). Significantly, however, when cells were cultured in the presence of reduced, yet still inhibitory, concentrations of PGF2α we observed a more pronounced rescue effect of the HIF-1α-specific shRNA on adipocyte differentiation. Thus, while PGF2α in a concentration range of 6.25-25 nM is still sufficient to markedly attenuate adipocyte differentiation, we found that expression of the HIF-1α-specific shRNA was able to significantly overcome the inhibitory effects of these concentrations of PGF2α on adipogenesis, as determined by both increased Oil Red O staining (Fig. 6D) and the expression of the adipocyte differentiation-specific marker proteins PPARγ, C/EBPα, and aP2 (Fig. 6E). Taken together, these results suggest that PGF2α mediates its inhibitory effects on adipocyte differentiation, at least in part, via the activation of the HIF-1 pathway.
Fig. 6.
Effects of shRNA-mediated HIF-1α knockdown on the ability of PGF2α to inhibit MDI-induced 3T3-L1 preadipocyte differentiation. A: Demonstration of decreased HIF-1α protein expression after HIF-1α shRNA-mediated knockdown. 3T3-L1 preadipocytes transduced with either control pSUPER retrovirus or the pSUPER-shHIF-1α retrovirus were treated with MDI in presence of 100 nM PGF2α. At day 2 post-differentiation nuclear extracts were prepared and analyzed by immunoblotting for expression of either HIF-1α or Sp1 as a control. B: RT-PCR analysis of DEC1 expression in either control or HIF-1α shRNA-transduced 3T3-L1 preadipocytes treated with MDI in the presence of 100 nM PGF2a for 0, 1, or 2 days. The expression of HPRT is shown as a control. C: Representative cell morphologies of either control or HIF-1α shRNA-transduced 3T3-L1 preadipocytes induced to undergo differentiation by the standard MDI protocol in the additional presence of 100 nM PGF2α for the first 48 h of the culture period. At 10 days post-differentiation cell morphology was analyzed by light microscopy. D: Effects of HIF-1α shRNA-mediated knockdown on the dose-dependent ability of PGF2α to inhibit adipocyte differentiation. 3T3-L1 preadipocytes transduced with either control pSUPER retrovirus or the pSUPER-shHIF-1α retrovirus were induced to undergo adipocyte differentiation by the standard MDI protocol in the presence of a range of PGF2α concentrations (6.25, 12.5, and 25 nM) present during the first 48 h of the culture period. After 10 days the extent of adipocyte differentiation was determined by Oil Red O-staining. E: Effects of HIF-1α shRNA-mediated knockdown on the ability of PGF2α to inhibit the expression of adipocyte specific marker proteins. 3T3-L1 preadipocytes transduced with either control pSUPER retrovirus or the pSUPER-shHIF-1α retrovirus were induced to undergo adipocyte differentiation by the standard MDI protocol in the presence of 12.5 nM PGF2α for the first 48 h of the culture period. After 10 days, whole cell extracts were prepared and the expression of C/EBPα, PPARγ, aP2 and actin was determined by immunoblotting. Data are representative of three independent experiments.
DISCUSSION
In the current study we have investigated the effects of the anti-adipogenic prostaglandin, PGF2α, on the activity of the HIF-1 transcription factor-signaling pathway during adipocyte differentiation. Although the HIF-1 signaling pathway is best known for its ability to be activated under conditions of hypoxia [Bruick, 2003], we find that this pathway is also markedly stimulated under normal oxygen conditions in differentiating 3T3-L1 preadipocytes treated with PGF2α. In fact, consistent with a recent report [Floyd et al., 2007], we find that there is a transient low level increase in HIF-1α protein levels in 3T3-L1 preadipocytes during the standard MDI-induced differentiation protocol. However, we find that treatment of these differentiating preadipocytes with PGF2α acts to significantly promote and enhance the sustained activation of the HIF-1 transcription factor signaling pathway, as measured by a marked increase in the nuclear levels of the HIF-1α polypeptide and a corresponding increase in the expression of the downstream HIF-1 target gene, DEC1 [Ivanova et al., 2001; Yun et al., 2002], a known inhibitor of adipocyte differentiation [Yun et al., 2002]. Moreover, we provide evidence that this PGF2α-induced normoxic activation of the HIF-1 transcription factor signaling pathway potentially plays a role in mediating the inhibitory effects of PGF2α on adipogenesis.
What accounts for this effect of PGF2α on the expression of HIF-1α under normoxic conditions? While the best known mechanism of HIF-1 regulation is undoubtedly the post-translational regulation of HIF-1α polypeptide stability [Bruick, 2003; Huang and Bunn, 2003], our data demonstrate that PGF2α does not utilize this common mechanism to increase HIF-1α levels, as we find that PGF2α treatment does not appear to stabilize the normal rapid protein turnover of the HIF-1α polypeptide. Rather, our data suggest that PGF2α acts to influence HIF-1α polypeptide expression by promoting the sustained expression of a specific HIF-1α mRNA isoform during the early stages of the differentiation process. Murine HIF-1α is encoded by two alternate mRNA isoforms that differ only in presence of two alternative first exons termed HIF-1α I.1 and HIF-1α I.2 [Wenger et al., 1997, 1998]. These two distinct mRNA isoforms are generated from the activities of two distinct promoters that are located 23 and 17 kb respectively upstream of the remaining common coding exons and result in the generation of two distinct HIF-1α polypeptides that are identical except for an additional N-terminal 12 amino acids present in the HIF-1α I.2 isoform. The HIF-1α I.1 exon-containing mRNA isoform has been reported to exhibit a tissue-specific and cell activation-dependent expression, whereas the I.2 exon-containing mRNA isoform is constitutively expressed in most cell types [Wenger et al., 1997, 1998]. Our data reveal that treatment of differentiating 3T3-L1 preadipocytes with PGF2α acts to selectively promote the sustained expression of the HIF-1α I.1 mRNA isoform. We find that the HIF-1α I.1 mRNA isoform is not expressed in growth-arrested 3T3-L1 preadipocytes prior to differentiation, but is transiently induced in response to MDI stimulation, which correlates nicely with the transient low level increase in HIF-1α protein levels that we observe at day 1 during normal MDI-induced adipocyte differentiation. This result is also consistent with the recent report of Floyd et al. [2007] who have also demonstrated that the HIF-1α I.1 mRNA and HIF-1α polypeptide are transiently induced by the standard MDI-induced 3T3-L1 differentiation protocol. Significantly, though, we find that treatment of differentiating 3T3-L1 preadipocytes with PGF2α acts to markedly prolong the expression of the HIF-1α I.1 mRNA, resulting in its continued expression throughout the entire first 3 days of the differentiation process, an effect that correlates with the increased effect of PGF2α on the expression of the HIF-1α protein that we observe. We also find that treatment of growth-arrested undifferentiated 3T3-L1 preadipocytes with PGF2α in the absence of MDI stimulation similarly induces the sustained expression of the HIF-1α I.1 mRNA (data not shown), indicating that this effect is intrinsic to the PGF2α signaling pathway and is not dependent upon the presence of any proadipogenic co-stimulatory signals or a particular stage of cellular differentiation. By way of contrast, we find that the HIF-1α I.2 mRNA isoform is expressed throughout all of the first 4 days of the differentiation process and its expression is not significantly influenced by the presence of PGF2α. Curiously, in contrast to the constitutive expression of the HIF-1α I.2 mRNA that we observe, Floyd et al. [2007] have reported that the HIF-1α I.2 mRNA is only transiently expressed during 3T3-L1 preadipocytes differentiation at days 3 and 4 post-MDI stimulation, the reason for this minor discrepancy is not currently clear. Nonetheless, taken together, our collective data are consistent with the notion that the increased expression of the HIF-1α polypeptide observed in PGF2α-treated differentiating 3T3-L1 preadipocytes under normoxic conditions is likely due to the selective enhancing effects of PGF2α on the sustained expression of the HIF-1α I.1 mRNA isoform. Indeed, a similar effect on the expression of the HIF-1α I.1 mRNA isoform has previously been reported to be responsible for the normoxic increase in HIF-1α levels and activity induced in T cell receptor-stimulated murine T cells [Lukashev et al., 2001].
HIF-1α has previously been demonstrated to play a key inhibitory role in the regulation of adipocyte differentiation under hypoxic conditions [Yun et al., 2002; Lin et al., 2006]. In this respect, exposure of undifferentiated preadipocyte cells to hypoxia has been shown to potently inhibit their MDI-induced differentiation into adipocytes [Sahai et al., 1994; Yun et al., 2002; Kim et al., 2005; Zhou et al., 2005; Lin et al., 2006; Floyd et al., 2007], whereas this inhibitory effect of hypoxia on adipogenesis is specifically abrogated in cells that are either genetically deficient in HIF-1α, or in which HIF-1α levels have been depleted by siRNA-mediated knockdown [Yun et al., 2002; Lin et al., 2006]. However, despite these compelling lines of evidence for a negative role of hypoxia and HIF-1 in the regulation of adipocyte differentiation, other reports have instead suggested that hypoxia may act to enhance the adipocyte-like phenotype under certain circumstances [Fink et al., 2004; Ren et al., 2006; Irwin et al., 2007], the reason for these contradictory findings are not clear, but maybe related to differences in either cellular context or environment. Others have also recently suggested that HIF-1 may even play a positive role during adipocyte differentiation [Floyd et al., 2007]. However, arguing against an absolute requirement for HIF-1 signaling during adipocyte differentiation is the observation that cells that are genetically deficient in HIF-1α are still able to readily undergo adipogenesis [Yun et al., 2002]. In fact, the recent observation that the targeted deletion of HIF-1α has been reported to actually enhance the adipogenic potential of adipose-derived adult stromal cells [Malladi et al., 2007], argues that the HIF-1 pathway is likely to act primarily as a negative regulator of adipogenesis.
Consistent with the negative regulatory role proposed for HIF-1α in the regulation of adipogenesis under hypoxic conditions [Yun et al., 2002; Lin et al., 2006], our current data now provide evidence that the HIF-1 pathway is also likely to play a role in mediating the inhibitory effects of PGF2α under normal oxygen conditions. First, our data show that exposure of differentiating 3T3-L1 preadipocytes to PGF2α results in the pronounced and sustained increase in the expression of HIF-1α and its downstream anti-adipogenic target gene DEC1, at a time known to be critical for commitment towards the mature adipocyte phenotype. Second, by using a constitutively active mutant form of HIF-1α, we also demonstrate that normoxic activation of HIF-1 is alone sufficient to inhibit the MDI-induced differentiation of 3T3-L1 preadipocytes by blocking their expression of the critical proadipogenic transcription factors C/EBPα and PPARγ. Third, we find that the shRNA-mediated knockdown of endogenous HIF-1α is able to both overcome the inhibitory effects of PGF2α on the expression of PPARγ,C/EBPα, and aP2, as well as rescue the ability of 3T3-L1 preadipocytes to undergo adipogenesis in the presence of inhibitory concentrations of PGF2α. Taken together, these observations suggest that the pronounced and sustained normoxic activation of the HIF-1 signaling pathway likely plays a key role in mediating the inhibitory effects of PGF2α on terminal adipocyte differentiation. We note, however, that the attenuation of PGF2α's inhibitory effects on adipogenesis by HIF-1α knockdown is not complete, and is most apparent only at reduced inhibitory concentrations of PGF2α. While these results might be explained by incomplete knockdown of HIF-1α expression, we believe that other PGF2α-induced pathways in addition to HIF-1 are also likely to contribute towards the inhibitory effects of PGF2α on adipogenesis. Nonetheless, despite this caveat, our data argue that the normoxic activation of the HIF-1 pathway is likely to play a prominent role in mediating the inhibitory effects of PGF2α on terminal adipocyte differentiation.
By what mechanism is the PGF2α-induced, normoxic activation of HIF-1 likely to inhibit adipogenesis? Previously, the hypoxic activation of HIF-1 has been shown to inhibit adipogenesis via the increased expression of the DEC1 transcriptional repressor, which has been shown to directly repress the expression of the PPARγ2 promoter [Yun et al., 2002]. Indeed, consistent with previous results [Yun et al., 2002], we find that ectopic expression of DEC1 is sufficient to potently block MDI-induced 3T3-L1 preadipocyte differentiation (data not shown). Hence, our observation that under normoxic conditions, PGF2α acts via HIF-1 to induce the expression of DEC1 coincident with its inhibitory effects on the expression of C/EBPα and PPARγ, provides a direct potential mechanistic basis to explain the inhibitory effects of PGF2α on adipogenesis. In this model, the PGF2α-induced expression of the transcriptional repressor DEC1 is proposed to block adipocyte differentiation by directly repressing the PPARγ2 promoter, thereby preventing the expression of this critical proadipogenic transcription factor that is known to be essential for the execution of the latter stages of terminal adipocyte differentiation. In addition to this potential direct inhibitory effect of DEC1 on PPARγ gene expression, another potential mechanism by which DEC1 could contribute towards the inhibition of adipogenesis is via the inhibition of the Bmal1 transcription factor (also known as MOP3). Bmal1 is a component of the molecular clock complex involved in the circadian rhythm-dependent regulation of gene expression [Bunger et al., 2000]. However, Bmal1 has also recently been shown to play a positive role in the regulation of adipogenesis [Shimba et al., 2005]. Bmal1 expression levels are increased during adipocyte differentiation and cells that are rendered deficient in Bmal1 are unable to undergo adipogenesis [Shimba et al., 2005]. Importantly, DEC1 is a known direct and specific inhibitor of Bmal1 activity [Honma et al., 2002]. Hence, the increased DEC1 expression levels that we observe following PGF2α treatment would be expected to antagonize Bmal1-dependent transcription and thereby attenuate its role in the adipogenic process. Thus, it appears that there are at least two non-mutually exclusive mechanisms by which PGF2α-induced, HIF-1-dependent expression of DEC1 could potentially act to attenuate adipocyte differentiation: direct repression of the PPARγ2 promoter and inhibition of Bmal1 activity. However, exactly what role DEC1 plays, or for that matter, whether DEC1 is the only critical downstream HIF-1 effector involved in the HIF-1-dependent inhibition of adipocyte differentiation remains to be seen.
In summary, our data afford significant new insights into the molecular mechanisms by which PGF2α acts to inhibit adipocyte differentiation. We have demonstrated that PGF2α promotes the sustained activation of the HIF-1 signaling pathway during 3T3-L1 preadipocyte differentiation under normal oxygen conditions and provide evidence that this normoxic activation of HIF-1 contributes towards the inhibitory effects of PGF2α on adipogenesis, most likely via the increased expression of the DEC1 transcriptional repressor.
ACKNOWLEDGMENTS
The authors thank Dr. Mary Hunzicker-Dunn for providing the caHIF-1α plasmid and Gregory Sabino for technical assistance.
Grant sponsor: National Institutes of Health; Grant number: DK-63298.
Abbreviations used
- PGF2α
prostaglandin F2α
- MDI
methylisobutylxanthine, dexamethasone and insulin
- C/EBP
CCAAT/enhancer-binding protein
- PPARγ
peroxisome proliferators-activated receptor γ
- HDAC
histone deacetylase
- HIF-1
hypoxia-inducible factor-1
- RT-PCR
reverse transcriptase-polymerase chain reaction
- MSCV
murine stem cell virus
- GFP
green fluorescent protein
- caHIF-1α
constitutively active HIF-1α
- ODD
oxygen-dependent degradation domain
- HPRT
hypoxanthine-guanine phosphoribosyl transferase
- shRNA
short hairpin RNA
REFERENCES
- Aminova LR, Chavez JC, Lee J, Ryu H, Kung A, Lamanna JC, Ratan RR. Prosurvival and prodeath effects of hypoxia-inducible factor-1alpha stabilization in a murine hippocampal cell line. J Biol Chem. 2005;280:3996–4003. doi: 10.1074/jbc.M409223200. [DOI] [PubMed] [Google Scholar]
- Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem. 2006;281:19881–19891. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, Kawahara R, Hauner H, Jain MK. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J Biol Chem. 2003;278:2581–2584. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: Implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- Bruick RK. Oxygen sensing in the hypoxic response pathway: Regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir DA, Miller CW, Ntambi JM. Preadipocyte differentiation blocked by prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation. 1996;60:203–210. doi: 10.1046/j.1432-0436.1996.6040203.x. [DOI] [PubMed] [Google Scholar]
- Chan DA, Sutphin PD, Denko NC, Giaccia AJ. Role of prolyl hydroxylation in oncogenically stabilized hypoxiainducible factor-1alpha. J Biol Chem. 2002;277:40112–40117. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Duplan E, Vigne P, Frelin C. Cyclosporin A prevents the hypoxic adaptation by activating hypoxiainducible factor-1alpha Pro-564 hydroxylation. J Biol Chem. 2003;278:15406–15411. doi: 10.1074/jbc.M211293200. [DOI] [PubMed] [Google Scholar]
- Davis KE, Moldes M, Farmer SR. The forkhead transcription factor FoxC2 inhibits white adipocyte differentiation. J Biol Chem. 2004;279:42453–42461. doi: 10.1074/jbc.M402197200. [DOI] [PubMed] [Google Scholar]
- Fink T, Abildtrup L, Fogd K, Abdallah BM, Kassem M, Ebbesen P, Zachar V. Induction of adipocyte-like phenotype in human mesenchymal stem cells by hypoxia. Stem Cells. 2004;22:1346–1355. doi: 10.1634/stemcells.2004-0038. [DOI] [PubMed] [Google Scholar]
- Floyd ZE, Kilroy G, Wu X, Gimble JM. Effects of prolyl hydroxylase inhibitors on adipogenesis and hypoxia inducible factor 1 alpha levels under normoxic conditions. J Cell Biochem. 2007;101:1545–1557. doi: 10.1002/jcb.21266. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA. The adipocyte: A model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280:E827–E847. doi: 10.1152/ajpendo.2001.280.6.E827. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Hirota K, Fukuda R, Takabuchi S, Kizaka-Kondoh S, Adachi T, Fukuda K, Semenza GL. Induction of hypoxia-inducible factor 1 activity by muscarinic acetylcholine receptor signaling. J Biol Chem. 2004;279:41521–41528. doi: 10.1074/jbc.M405164200. [DOI] [PubMed] [Google Scholar]
- Honma S, Kawamoto T, Takagi Y, Fujimoto K, Sato F, Noshiro M, Kato Y, Honma K. Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature. 2002;419:841–844. doi: 10.1038/nature01123. [DOI] [PubMed] [Google Scholar]
- Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–19578. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- Irwin R, LaPres JJ, Kinser S, McCabe LR. Prolylhydroxylase inhibition and HIF activation in osteoblasts promotes an adipocytic phenotype. J Cell Biochem. 2007;100:762–772. doi: 10.1002/jcb.21083. [DOI] [PubMed] [Google Scholar]
- Ivanova AV, Ivanov SV, Danilkovitch-Miagkova A, Lerman MI. Regulation of STRA13 by the von Hippel-Lindau tumor suppressor protein, hypoxia, and the UBC9/ubiquitin proteasome degradation pathway. J Biol Chem. 2001;276:15306–15315. doi: 10.1074/jbc.M010516200. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Kim KH, Song MJ, Chung J, Park H, Kim JB. Hypoxia inhibits adipocyte differentiation in a HDAC-independent manner. Biochem Biophys Res Commun. 2005;333:1178–1184. doi: 10.1016/j.bbrc.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxiainducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepak NM, Serrero G. Inhibition of adipose differentiation by 9 alpha, 11 beta-prostaglandin F2 alpha. Prostaglandins. 1993;46:511–517. doi: 10.1016/0090-6980(93)90070-n. [DOI] [PubMed] [Google Scholar]
- Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- Liu L, Clipstone NA. Prostaglandin F2alpha inhibits adipocyte differentiation via a Galphaq-Calcium-Calcineurin-Dependent signaling pathway. J Cell Biochem. 2007;100:161–173. doi: 10.1002/jcb.21044. [DOI] [PubMed] [Google Scholar]
- Lukashev D, Caldwell C, Ohta A, Chen P, Sitkovsky M. Differential regulation of two alternatively spliced isoforms of hypoxia-inducible factor-1 alpha in activated T lymphocytes. J Biol Chem. 2001;276:48754–48763. doi: 10.1074/jbc.M104782200. [DOI] [PubMed] [Google Scholar]
- MacDougald OA, Mandrup S. Adipogenesis: Forces that tip the scales. Trends Endocrinol Metab. 2002;13:5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Hypoxia inducible factor-1alpha deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue Eng. 2007;13:1159–1171. doi: 10.1089/ten.2006.0265. [DOI] [PubMed] [Google Scholar]
- Neal JW, Clipstone NA. Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J Biol Chem. 2002;277:49776–49781. doi: 10.1074/jbc.M207913200. [DOI] [PubMed] [Google Scholar]
- Ng HH, Bird A. Histone deacetylases: Silencers for hire. Trends Biochem Sci. 2000;25:121–126. doi: 10.1016/s0968-0004(00)01551-6. [DOI] [PubMed] [Google Scholar]
- Otto TC, Lane MD, Cox MM. Adipose development: From stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- Page EL, Robitaille GA, Pouyssegur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- Pear WS. Transient transfection methods for preparation of high-titer retroviral supernatants. In: Ausbel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. John Wiley and Sons, Inc.; New York: 2003. [DOI] [PubMed] [Google Scholar]
- Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao L, Jia M, Cai H, Han ZC, Yang R, Chen G, Zhao RC. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12–21. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Ross DA, Hannenhalli S, Tobias JW, Cooch N, Shiekhattar R, Kadesch T. Functional analysis of Hes-1 in preadipocytes. Mol Endocrinol. 2006;20:698–705. doi: 10.1210/me.2005-0325. [DOI] [PubMed] [Google Scholar]
- Sahai A, Patel MS, Zavosh AS, Tannen RL. Chronic hypoxia impairs the differentiation of 3T3-L1 fibroblast in culture: Role of sustained protein kinase C activation. J Cell Physiol. 1994;160:107–112. doi: 10.1002/jcp.1041600113. [DOI] [PubMed] [Google Scholar]
- Serrero G, Lepak NM, Goodrich SP. Prostaglandin F2 alpha inhibits the differentiation of adipocyte precursors in primary culture. Biochem Biophys Res Commun. 1992;183:438–442. doi: 10.1016/0006-291x(92)90500-k. [DOI] [PubMed] [Google Scholar]
- Shi X, Shi W, Li Q, Song B, Wan M, Bai S, Cao X. A. glucocorticoid-induced leucine-zipper protein, GILZ, inhibits adipogenesis of mesenchymal cells. EMBO Rep. 2003;4:374–380. doi: 10.1038/sj.embor.embor805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Sun H, Taneja R. Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc Natl Acad Sci USA. 2000;97:4058–4063. doi: 10.1073/pnas.070526297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Dalgin G, Xu H, Ting CN, Leiden JM, Hotamisligil GS. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science. 2000;290:134–138. doi: 10.1126/science.290.5489.134. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxiainducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger RH, Rolfs A, Kvietikova I, Spielmann P, Zimmermann DR, Gassmann M. The mouse gene for hypoxia-inducible factor-1alpha-genomic organization, expression and characterization of an alternative first exon and 5' flanking sequence. Eur J Biochem. 1997;246:155–165. doi: 10.1111/j.1432-1033.1997.t01-1-00155.x. [DOI] [PubMed] [Google Scholar]
- Wenger RH, Rolfs A, Spielmann P, Zimmermann DR, Gassmann M. Mouse hypoxia-inducible factor-1alpha is encoded by two different mRNA isoforms: Expression from a tissue-specific and a housekeepingtype promoter. Blood. 1998;91:3471–3480. [PubMed] [Google Scholar]
- Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: A mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-beta/Smad3 signaling. J Biol Chem. 2005;280:22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]