Abstract
Full details are provided for the total synthesis of several members of the hapalindole family of natural products, including hapalindole Q, 12-epihapalindole D, 12-epi-fischerindole U, 12-epi-fischerindole G, 12-epi-fischerindole I, and welwitindolinone A. Use of the recently developed direct indole coupling enabled an efficient, practical, scaleable, and protecting group-free synthesis of each of these natural products. The original biosynthetic proposal is reviewed, and a revised biosynthetic hypothesis is suggested, validated by the above syntheses. The syntheses are also characterized by an adherence to the concept of “redox economy”. Analogous to “atom economy” or “step economy”, “redox economy” minimizes the superfluous redox manipulations within a synthesis; rather, the oxidation state of intermediates linearly and steadily increases throughout the course of the synthesis.
Keywords: total synthesis, hapalindole, fischerindole, welwitindolinone, oxidative coupling, protecting group-free
Introduction
The first members of the hapalindole-type natural products were isolated from the Stigonemataceae family of cyanobacteria in 1984 by Moore and colleagues.1 In the 24 years since their initial discovery, 63 members2 have been added to this family of alkaloids, which include the hapalindoles, fischerindoles, welwitindolinones, ambiguines, hapalindolinones, hapaloxindoles, and fontonamides (see Chart 1). These natural products have been isolated from soil samples in a myriad of habitats around the globe,3 and a broad range of biological activities arises from the different structural classes. Insecticidal activity is observed for several hapalindoles2l and welwitindolinones.2f Reports have surfaced of antialgal activity from the hapalindoles,1 antimycotic activity from the hapalindoles,1,2e,i welwitindolinones,2f and ambiguines,2d,k and antibacterial activity from the hapalindoles2m,4 and ambiguines.2k Additionally, it has been found that hapalindolinone A inhibits arginine vasopression binding.2b Finally, potent anticancer activity against multiple drug resistant ovarian cancer cell lines has been reported for the welwitindolinones,2f,5 apparently exerting this effect through microtubule depletion.6
Chart 1.
All known hapalindole-type natural products.
Not only do a vast number of the members of this natural product family exhibit potent and exciting biological activities, but they also all contain intriguing and unprecedented molecular architectures. Though distinct, they are united by several structural features, most notably an indole (or indole-derived) heterocycle with a monoterpene unit appended at C(3) (see Scheme 1 for numbering), comprising the core of these molecules. All but a handful of them contain an isonitrile or an isothiocyanate at C(11), with an all-carbon quaternary center, comprised of a methyl and vinyl group, vicinal to this moiety (C(12)). The hapalindoles are the simplest members of this family, containing the core structure described above, housed within a tricyclic framework. Many contain further functionalization, either in the form of unsaturation (at C(10)) or chlorination (at C(13)), and several contain an additional carbocycle arising from the union of C(4) of indole with the isopropylidene unit at C(15). The fischerindoles, tetracycles formed via the union of C(2) of indole with the isopropylidene unit at C(15), are also characterized by varying degrees of functionalization and oxidation. The hapaloxindoles and fontonamides are structurally related to the tetracyclic hapalindoles, although the indole has been either oxidized to give the oxindole or oxidatively cleaved to form the formylkynurenine. Also related to the hapalindoles, albeit much more complex, are the ambiguines, which contain additional functionalization at C(2) of indole, specifically a tert-prenyl moiety. In the most complex ambiguines, this tert-prenyl group is further cyclized and oxidized. In addition to the unifying structural features of this family of natural products, the hapalindolinones contain a unique component within their molecular architecture, specifically a spirocyclic cyclopropane that joins C(11) with C(3) of the oxindole heterocycle. Finally, the welwitindolinones are found in one of two structural classes, the first being welwitindolinone A, which contains a spirocyclic cyclobutane centered around C(3). The remaining welwitindolinones are comprised of a [4.3.1]-bicyclononanone core, which contains an assortment of oxidative functionalization.
Scheme 1.
Moore's proposed biosynthetic relationships between the hapalindole-type alkaloids; [O] = oxidation, [S] = sulfur insertion.
With a family of such diverse and unique molecular architectures, it should come as no surprise that several syntheses have been reported for these natural products, specifically focusing on the simpler members of the family. Syntheses have been reported for hapalindoles G,7 H,8 J,9 M,9 Q,10 O,11 and U,8 in addition to approaches to various other hapalindoles.12 There are no reported efforts towards the hapalindolinones, hapaloxindoles, and fontonamides and only one approach towards an ambiguine.13 The first total synthesis of an ambiguine (ambiguine H) was reported from our laboratory.14 Despite the many approaches toward the welwitindolinones,15 at the time of our initial communication16 of the work presented herein, the members of the welwitindolinone family had not yet succumbed to synthesis; however, the Wood group reported a very elegant synthesis of welwitindolinone A shortly thereafter.17
Biosynthetic Relationships and Retrosynthetic Analysis
Biomimetic syntheses are often more efficient due to the tactics that Nature employs, namely rapid assembly of skeletal complexity, a linear increase of oxidation state, use of mild and simple reagents, and the ability to control chemoselectivity (lack of protecting groups).18 Despite its inherent advantages, biomimetic syntheses can be exceedingly difficult, due to the inability of chemists to attain the chemo-, regio-, and stereocontrol characterizing most enzymatic processes. The careful practitioner can make use of many abiotic tools in solving these problems; however, these methods usually demand significant departure from the ideal biomimetic route. In light of these difficulties, it is certainly possible, and perhaps prudent, to find an appropriate balance when designing a retrosynthesis. An ideal synthesis might entail the use of powerful synthetic methods, coupled with a flexible adherence to the general synthetic blueprint provided by Nature.
Given these considerations, and with such a large and diverse family of complex natural products, a biosynthetic proposal that comprehensively describes the interrelationships between each of the members would undoubtedly be enlightening to any synthetic undertaking. The Moore group, in conjunction with their elegant isolation studies, put forth many plausible biosynthetic ruminations that are summarized in Scheme 1. Moore's biosynthesis begins with the tryptophan derivative (1a) and terpene (1b), which are enzymatically joined via chloronium-promoted polyolefin cyclization to provide the tricyclic hapalindole core (i.e., 12-epi-hapalindole E (2), Scheme 1). At this point, Moore and co-workers proposed that the tricycle can proceed through multiple divergent pathways; the first of which (Path A) commences with a cyclization between C(4) of indole and the isopropylidene unit at C(15), leading to the tetracyclic hapalindoles (i.e., 12-epi-hapalindole G (3)). These natural products can then undergo further oxidation at the indole moiety, leading to the oxindole (i.e., anhydrohapaloxindole A (4)), which can be oxidatively cleaved to give the formylkynurenine (i.e., hapalonamide V (5)). Alternatively, the tetracyclic hapalindoles can have a tert-prenyl moiety attached to C(2) (i.e., ambiguine A (6)), which can be engaged in an intramolecular cyclization, leading to the pentacyclic ambiguines (i.e., ambiguine E (7)). These alkaloids can then be further oxidized (i.e., ambiguine D (8)) or rearranged (i.e., ambiguine G (9)). Furthermore (Path B), the tricyclic hapalindoles can undergo cyclization between C(2) and the isopropylidene at C(15), providing the fischerindoles (i.e., 12-epi-fischerindole G (10)). Finally, Moore and co-workers proposed that the tricyclic hapalindoles can be oxidized to give the putative intermediate 11, which has not been isolated as a natural product (Path C). They further proposed that this intermediate undergoes an acid-catalyzed cyclization to afford welwitindolinone A (12), presumably in the pocket of an enzyme. If 12 could be further oxidized, leading to the intermediate epoxide 13, the [4.3.1]bicyclononane system could be formed after rearrangement (i.e., “welwitindolinone B isonitrile” (14a), which is likely to be a natural product that has yet to be isolated). Moore and co-workers suggested that methylation and oxidation of 14a could lead to N-methylwelwitindolinone C (15) and further demonstrated the conversion of 15 into 3-hydroxy-N-methylwelwitindolinone C (17) and N-methylwelwitindolinone D (19).
While the Moore biosynthetic hypothesis provides an adequate explanation of the possible relationships between many of the distinct structural classes, a few points remained uncertain, primarily relating to the formation of the welwitindolinones. First, the proposal that 12 arises from the unsaturated intermediate 11 via an acid-catalyzed cyclization seems unlikely. The fact that 63 members of this natural product family have been isolated, while 11 has not been one of them, casts doubt on whether this compound is a plausible intermediate. Given the stability that 11 should demonstrate, at least trace quantities of this compound would be expected in the isolation broths. More importantly, there seems to be little thermodynamic driving force for the conversion of 11 into 12, due to the generation of a strained spirocyclobutane in this transformation. Second, the mechanistic explanation for the conversion of welwitindolinone A (12) into “welwitindolinone B isonitrile” (14a) lacks proper literature precedent. Third, the proposal that N-methylwelwitindolinone C (15) arises from remote oxidation of 14a could conceivably be explained by an alternate hypothesis. Finally, the isolation literature lacks a biosynthetic hypothesis to account for the genesis of the hapalindolinones.
Given the concerns delineated above, an alternative biosynthetic hypothesis is proposed in Scheme 2. Rather than arising from hypothetical metabolite 11, welwitindolinone A (12) could arise from an oxidative ring contraction of 12-epi-fischerindole I (21); the latter could be formed via a benzylic oxidation of 12-epi-fischerindole G (10), which could in turn arise from the tricyclic hapalindole 12-epi-hapalindole E (2). Furthermore, an alternative, albeit untested, mechanistic hypothesis for the conversion of 12 into “welwitindolinone B isonitrile” (14a) is put forth, which is more in line with literature precedence. Isonitriles are relatively electron-withdrawing, inductively stabilizing negative charges, and have not been invoked as electron-donating entities.19 It is therefore unlikely that the isonitrile would participate in a fracture of the epoxide via electron donation that would lead to a fissure of the cyclobutane ring. Alternatively, based on evidence gleaned in this laboratory,20 it is more reasonable to invoke electron donation from the N(1) lone pair through the aromatic ring to break the cyclobutane, leading to the α-isocyanoketone enolate (22). This enolate could then attack the highly unstable, extremely electrophilic azaorthoquinodimethane generated in the reaction, leading to 14a after tautomerization.21 Additionally, the remote oxidation of 14a to N-methylwelwitindolinone C (15), although certainly possible with enzymatic intervention, is unlikely. Rather, if 21 were to undergo allylic oxidation, intermediate 23 could be accessed. Upon oxidative ring contraction, similar to that proposed for 12, the direct product would contain an unstable cyclobutane with vicinal exocyclic olefins. The trisubstituted olefin might then isomerize to form the vinyl chloride (24), thus alleviating this additional strain on the cyclobutane. Oxidative ring expansion of 24 and methylation of the indole nitrogen atom could then lead directly to 15. Finally, we propose that the hapalindolinones could arise from an oxidative coupling event between the C(3) and C(11) carbons of an appropriate tricyclic hapalindole (i.e. 12-epi-hapalindole Q (25)), generating the oxindole with the spirocyclopropane moiety (i.e. hapalindolinone B (26)).
Scheme 2.
Alternative biosynthetic proposal.
With a compelling biosynthetic hypothesis in hand, attention could be turned toward designing a synthesis of welwitindolinone A (12). However, before such a task was undertaken, it seemed prudent to first consider a synthesis of simpler members of the family, such as hapalindole Q (27) and 12-epi-fischerindole U (29). It was envisioned that such efforts would reveal hidden clues into the fundamental reactivity of these alkaloids and that they would help develop a general route by which to access the core structure. Hapalindole Q (27) had been synthesized previously by the Albizati (eight steps, 7.2% overall yield)10b and Kerr (twelve steps, 1.5% overall yield)10c,d groups, but the synthesis of 29 had not been reported prior to our initial communication of this work.10a Since a route to 12 was the ultimate goal, a more efficient route to the core (i.e., 27 and 29) of this alkaloid was required (Scheme 3). Although a cyclization of 27 could certainly be investigated to form 29, this transformation was instead planned at the ketone stage (28), given the acid-sensitivity of the isothiocyanate group. As such, both natural products could be traced back to their respective ketone analogues 28 and 30, the latter of which should be accessible from the former via an acid-catalyzed cyclization. Ketone 28 can be further simplified to the indole/carvone adduct 31 through straightforward functional group transformations. At this stage, a strictly biomimetic synthesis would require a chloronium-promoted polyolefin cyclization to install the terpene moiety; however, a potentially more direct and powerfully simplifying transformation would involve direct formation of the key C(3)-C(10) bond.
Scheme 3.
Retrosynthetic analysis of hapalindole Q (27) and 12-epi-fischerindole U (29).
Total Synthesis of Hapalindole Q
Several strategies were investigated for the synthesis of the requisite indole/carvone adduct (31) before a method was finally developed to successfully provide the desired material. Initially, it was reasoned that 31 could be accessed from an aldol reaction with subsequent reduction to form the requisite indole moiety (Scheme 4). Indeed, quenching the enolate of carvone with MOM-protected isatin (32) provided the desired aldol product (33) in excellent yield. Unfortunately, reduction of this intermediate proved to be problematic. All methods investigated to directly reduce this compound to indole 37 (e.g., LiAlH4, BH3•THF) were met with failure, so a stepwise solution was sought. Assuming that the C(3) hydroxyl group was preventing reduction of the oxindole, various deoxygenation conditions (e.g., Barton,22 CS(imid)2/hν23) were brought to bear for the removal of this alcohol (38) prior to indole reduction; however, all attempts were unsuccessful.24 Reasoning that elimination of the hydroxyl moiety would provide an intermediate upon which further reductions could be performed, various conditions were screened to elicit dehydration (e.g., Martin sulfurane,25 MsCl/base, TFA/TFAA) before Burgess reagent26 successfully provided the enone (36). However, the yield of this process was irreparably dismal, precluding further chemistry. Since the alcohol could not be reduced, removed, or efficiently eliminated, methods to exchange this moiety for a chlorine atom, which could theoretically be removed with greater facility, were investigated. Employing the conditions developed by Nicolaou,27 33 was dissolved in thionyl chloride, providing the cyclic ether 35 (colorless cubes, mp 166-168 °C, see Scheme 4 for X-ray crystallographic analysis) and the unexpectedly stable, semi-deprotected compound 34, which lead to a dead-end once again.
Scheme 4.
Attempted synthesis of hapalindole Q via an aldol reaction. Reagents and conditions: (a) Carvone, LDA (1.0 equiv), THF, -78 °C, 30 min; then 32 (0.83 equiv), 30 min, -78 to 23 °C, 92%; (b) SOCl2, 23 °C, 100 min, 34: 44% and 35: 37%; (c) Burgess reagent (2.0 equiv), PhH, 50 °C, 3 h, 12%; LDA = lithium diisopropylamide, THF = tetrahydrofuran, PhH = benzene.
The resistance of intermediate 33 to reduction necessitated the investigation of alternate means to forge the key bond in 31. Since removal of the tertiary alcohol had proved to be an intractable problem in the previous route, an approach that circumvented the generation of this group was sought, leading to an oxidative enolate coupling approach to form the desired bond (Scheme 5). The oxidative dimerization of carbonyl compounds has been known for more than 70 years; however, it has yet to become widely utilized by the synthetic community. This reaction has been reviewed,28 most recently during the full account of the oxidative indole coupling reaction developed in our laboratory,29 and therefore this information will not be reviewed here. Even though a myriad of literature procedures concerning oxidative enolate couplings was available, many potential pitfalls could be encountered while pursuing this route. By the inception of this work, only one example of an oxindole oxidative dimerization had been reported.30a Additionally, in order to achieve high yields of heterodimerized products in an oxidative enolate coupling, three or more equivalents of one coupling partner were usually required.30b,c Finally, few investigations into the factors that govern the heterodimerization event had been performed; thus, it was unknown whether any of the desired heterocoupled product would be obtained. Despite such potential pitfalls, an examination of the oxidative oxindole coupling was undertaken, which would, at the minimum, provide further insight into the elements that govern such transformations.
Scheme 5.
Oxindole oxidative coupling route to hapalindole Q. Reagents and conditions: (a) Carvone (1.0 equiv), 39 (1.0 equiv), LDA (2.1 equiv), THF, -78 °C, 30 min; then oxidant (2.0 equiv), 23 °C, 20 min; LDA = lithium diisopropylamide, THF = tetrahydrofuran, [O] = oxidation.
As a starting point for the examination of this heterocoupling reaction, treatment of carvone and MOM-protected oxindole (39)31 with FeCl3 in DMF provided the desired product (40) in ca. 15% yield. Given the success of this coupling, an optimization of this particular reaction was undertaken, centering primarily on oxidant selection (Table 1). Hypervalent iodine and cupric-based oxidants were less efficient at promoting the coupling than the ferric-based systems, so the focus of further studies was thus narrowed. Success was finally realized when the acetylacetonate-type ligands were utilized on the iron center. Specifically, Fe(t-BuCOCHCOCH3)3 was exceptionally efficient, providing an 83% isolated yield of the desired product (as a 1:1 mixture of diastereomers at C(3)), utilizing only equimolar quantities of both coupling partners! These results have led to the hypothesis that careful tuning of the oxidant's oxidation potential to more closely match one of the coupling partners could lead to selective heterodimerizations.32 With high-yielding access to intermediate 40, efforts were turned towards the reduction of oxindole 40 to indole 37 (Scheme 5). Once again, the reduction proved recalcitrant, necessitating yet another reevaluation of the synthetic strategy.
Table 1.
Optimization of the oxidative oxindole coupling; LDA = lithium diisopropylamide, [O] = oxidation.
| Oxidant | Yield |
|---|---|
| Phl(OAc)2 | 10% |
| Cu(2-ethylhexanoate)2 | 15% |
| FeCl3 | ca 15% |
| Fe(PhCOCHCOCH3)3 | 30% |
| Fe(CH3COCHCOCF3)3 | 40% |
| Fe(C10H7COCHCOCF3)3 | 40% |
| Fe(CH3COCHCOCH3)3 | 45% |
| Fe(t-BuCOCHCOCH3)3 | 83% |
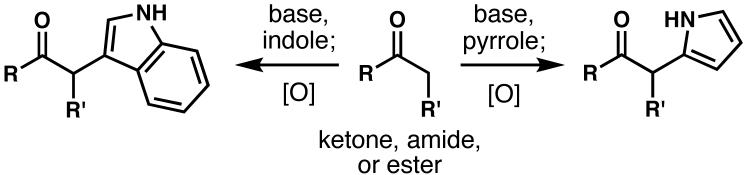
As with the aldol route, an alternative to the oxindole coupling was sought: one that would avoid a problematic reduction step. As such, the most straightforward method by which to circumvent this difficulty would be to directly attach the indole to the carvone moiety. Unfortunately, no such method had been reported, requiring the invention of chemistry to fill this gap. Inspired by the oxidative coupling literature, in conjunction with Barton's classic synthesis of usnic acid,33 the oxidative indole coupling was conceived. In this reaction,10a,29 an indole can be directly attached to a variety of carbonyl compounds in good yields (Scheme 6). The method was found to generate the desired indole/carvone adduct (31, colorless cubes, mp 129-130 °C, structure verified by X-ray crystallographic analysis) in good yield and in one synthetic operation from commodity starting materials (Scheme 7); this has also been extended to the coupling of unfunctionalized pyrroles.34
Scheme 6.
Oxidative indole and pyrrole couplings.
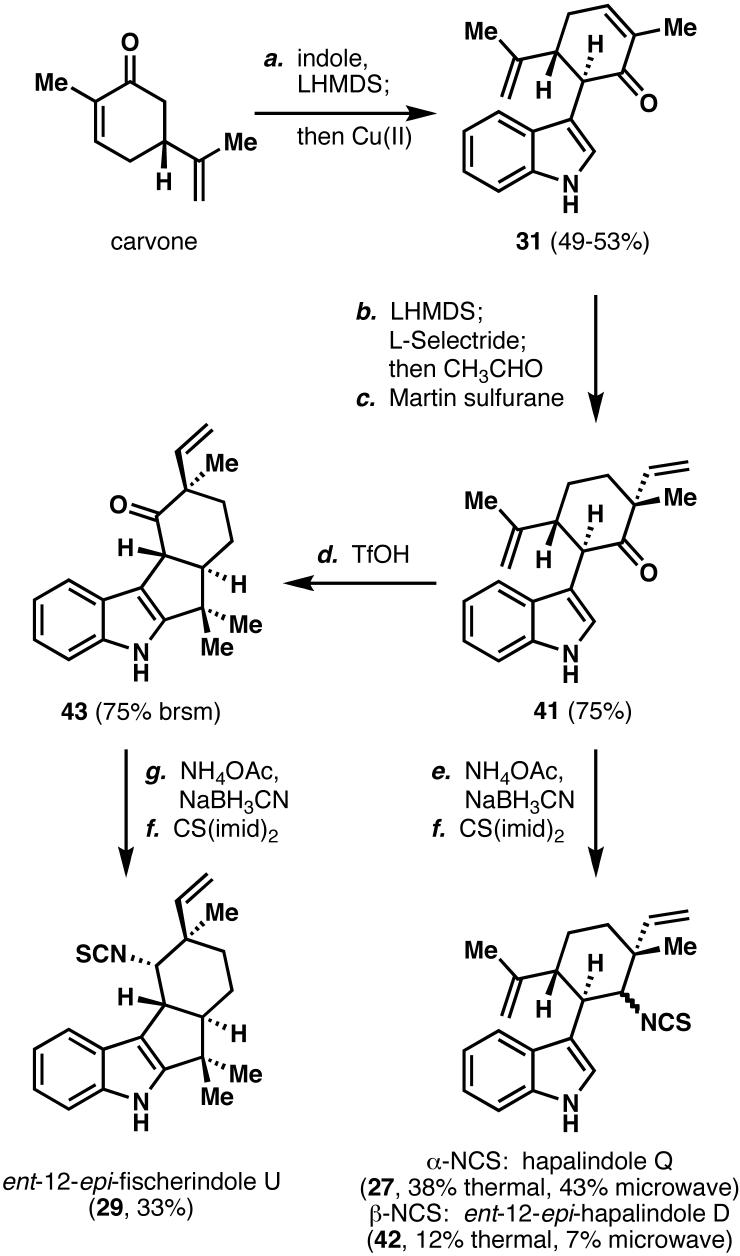
Scheme 7.
Total syntheses of hapalindole Q (27), ent-12-epi-hapalindole D (42), and ent-12-epi-fischerindole U isothiocyanate (29). Reagents and conditions: (a) Indole (2.0 equiv), carvone (1.0 equiv), LHMDS (3.3 equiv), THF, -78 °C, 30 min; then copper(II)2-ethylhexanoate (1.5 equiv), -78 to 23 °C, 15 min, 49-53%; (b) LHMDS (1.5 equiv), THF, -78 °C, 20 min; then L-Selectride (1.05 equiv), 1 h, then CH3CHO (6.0 equiv), -78 to 23 °C, 2 h; (c) Martin sulfurane (1.1 equiv), CHCl3, 10 min, 75% (2 steps); (d) TMSOTf (3 equiv), MeOH (1.1 equiv), DCM, 0 °C, 1 h, 31% isolated, 75% brsm; (e) NaCNBH3 (10 equiv), NH4OAc (40 equiv), MeOH, THF, microwave, 150 °C, 2 min, 61% combined; (f) CS(imid)2 (1.1 equiv), DCM, 0 to 23 °C, 3 h; (g) NaCNBH3 (10 equiv), NH4OAc (40 equiv), MeOH, THF, 23 °C, 7 d, 55%; LHMDS = lithium hexamethyldisilazide, THF = tetrahydrofuran, TMSOTf = trimethylsilyl trifluoromethanesulfonate, DCM = dichloromethane.
With gram quantities of 31 in hand, attention was turned to the completion of the total synthesis of 27 and 29, which first required reductive alkylation of the C(12)-C(13) olefin.35 Treatment of 31 with two equivalents of L-Selectride was expected to first deprotonate N(1)-H, then perform a conjugate reduction to generate the enolate, which could subsequently be trapped with acetaldehyde. Surprisingly, 1,4-hydride addition preceded N(1)-H deprotonation, thus generating the enolate, which in turn deprotonated N(1), forming the corresponding reduced enone (see Chart 2). Rather than protecting the indole nitrogen atom, N(1)-H was first deprotonated with LHMDS, followed by addition of L-Selectride, and the resulting enolate was trapped with acetaldehyde. This intermediate alcohol was immediately dehydrated with Martin sulfurane to give vinylated compound 41, which intersected the Albizati synthesis.10b Reductive amination using Albizati's conditions (NH4OAc, NaCNBH3, RT, 7 days) provided the desired amine in 61% yield as a 3:1 mixture of diastereomers. Alternatively, microwave irradiation (150 °C, 2 min) provided an identical yield of the product, with an increased diastereomeric ratio of 6:1. To the best of our knowledge, this is a unique example of an increase in diastereoselectivity as a consequence of microwave irradiation. Treatment of the resulting amine with CS(imid)2 installed the isothiocyanate, thus completing the total synthesis of 27 and the first total synthesis of ent-12-epi-hapalindole D (42). Tricyclic ketone 41 could also undergo a biomimetic7 acid-catalyzed cyclization to provide the tetracyclic ketone 43 upon exposure to in situ-generated triflic acid.36 Reductive amination and formation of the isothiocyanate completed the first total synthesis of 29, which also allowed the determination of the absolute stereochemistry of the fischerindole-type natural products (i.e., opposite to that depicted in Scheme 7). The syntheses of 27, 42, and 29 proceeded enantiospecifically in 15%, 4.8%, and 9.8% overall yields respectively, without resorting to protecting groups or superfluous redox manipulations.10a
Chart 2.

Reductive alkylation byproduct.
Retrosynthetic Analysis of Welwitindolinone A
Having demonstrated that the direct indole coupling could enable rapid access to several of the simpler members of the hapalindole family of natural products, attention could then be turned to the more complex members. Welwitindolinone A (12) was an attractive target for total synthesis due to its strikingly unique molecular architecture. It contains three all-carbon quaternary centers, two of which are chiral, and an asymmetrically disposed neopentyl chlorine atom, all incorporated into a highly strained spirocyclobutane-containing oxindole. As already discussed (vide supra), the revised biosynthetic hypothesis proposes that 12-epi-fischerindole I (21) is converted into 12 via oxidative ring contraction.37 Such ring contractions are primarily utilized for the preparation of spirocyclic 5-membered rings from annulated 6-membered rings and often require relatively harsh reaction conditions to proceed.38 Only one example has been reported for the direct preparation of a spirocyclic 4-membered ring from an annulated 5-membered ring;39 however, a related conversion has been observed in this laboratory to generate a strained β-lactam (see Chart 3).20 Furthermore, it is conceivable that 21 could arise from 12-epi-fischerindole G (10) via benzylic oxidation (Scheme 8). Through straightforward functional group manipulations, 10 could be derived from the tetracyclic ketone 44, which in turn could arise from an acid-catalyzed cyclization of tricyclic ketone 45. Exploiting the direct coupling methodology developed for the synthesis of 27, 45 could be obtained from the coupling of indole with 46. Chloroketone 46 was a new chemical entity; however, a very similar analogue had been prepared by Fukuyama en route to hapalindole G.7 Fukuyama's chloroketone (see Chart 4) is diastereomeric at C(12) and the chemistry utilized for its preparation was unfortunately not amenable for the preparation of 46.
Chart 3.

Strained β-lactam in chartelline C.
Scheme 8.
Retrosynthetic analysis of welwitindolinone A (12).
Chart 4.

Fukuyama's chloroketone: diastereomer of 46 at C(12).
Total Synthesis of ent-Fischerindole G
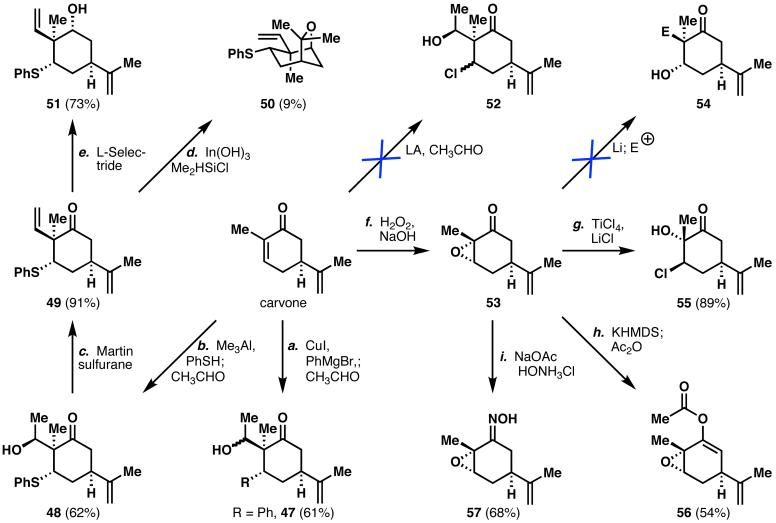
Historically, installation of chlorine atoms adjacent to quaternary centers has been met with difficulty and it was anticipated that 46 would not be an exception.40 Forays into the preparation of this compound focused on direct means for concurrent installation of the chlorine atom and the quaternary stereocenter. As such, attempts were made to apply a modified Baylis-Hillman-type reaction41 in which β-chloroketones are generated from the corresponding enones (Scheme 9), with concomitant incorporation of an aldehyde. However, this reaction was never utilized to install a quaternary center at the α-position prior to this work. Unfortunately, none of the desired product (52) was obtained using these conditions. Given the limited prospects for direct installation of the hallmark chlorine atom, attention was instead focused on a variety of chlorine equivalents. Carboxylic acids can be readily converted into chlorines via a Hunsdiecker reaction, so several acyl anion equivalents [e.g., 1,3-dithiane derivatives, (MeO)3CH, (PhS)3CH] were examined in 1,4-additions to carvone, but none of them provided the desired product. Encouragingly, successful 1,4-addition was observed with phenylcuprate and the incipient enolate was trapped with acetaldehyde to give 47. However, attempts to oxidatively degrade the aromatic ring42 led to significant over-oxidation. A thiophenyl group was next targeted as a chlorine equivalent, due to the propensity of such moieties to undergo α-chlorination or Pummerer rearrangements. Thiophenol participated in an efficient vicinal difunctionalization of carvone with aluminum catalysis43 to provide, after dehydration with Martin sulfurane, thioether 49. Attempts to directly chlorinate 49 were unsuccessful using a variety of reagents (e.g., NCS, Raney Ni/CCl4, SO2Cl2, CCl4/hν, Cl3CCOCCl3). Chlorination with concomitant removal of the thiol under numerous conditions (e.g., PIFA/LiCl, LiNaphthalenide/TsCl, TiCl4/CCl4, MeI/NaCl4/CCl4, MeI/NaCl44) was also investigated, but to no avail. Routes in which the ketone was first converted into the chloride, followed by sulfide oxidation, were also considered. Direct reductive chlorination of the ketone,45 instead of providing the desired product, led to the unexpected bicycle 50. Attempts to generate the vinyl chloride via POCl3, which could potentially be carefully hydrogenated to the alkyl chloride, were also fruitless. Unable to perform a direct reductive chlorination of the carbonyl, 49 was first reduced to the neopentyl alcohol (51), then unsuccessfully subjected to a variety of chlorination conditions (SOCl2/base, PPh3/CCl4,46 TCT/DMF47). These chlorinations presumably failed due to the extremely hindered nature of this particular alcohol. Before further effort was expended on this chlorination, the Pummerer rearrangement of thioether 49 was investigated to determine its feasibility in this system, which revealed that the reaction was not possible under a variety of conditions (IBX,48 m-CPBA,49 H2O2,50 tBuOOH, PhI(OAc)2).
Scheme 9.
Failed attempts to synthesize the key chloroketone. Reagents and conditions: (a) CuI (1.0 equiv), THF, PhMgBr (1.1 equiv), -78 to 0 to -78 °C, 1 h; then carvone (1 equiv), 30 min; then CH3CHO (2.0 equiv), -78 to 23 °C, 30 min, 61%; (b) Me3Al (1.2 equiv), DCM, PhSH (1.2 equiv), 0 °C, 20 min; then carvone (1.0 equiv), -78 °C, 15 min; then THF, CH3CHO (1.2 equiv), 20 min, 62%; (c) CCl4, Martin sulfurane (1.1 equiv), 91%; (d) In(OH)3 (0.05 equiv), CHCl3, Me2HSiCl (1.2 equiv), 23 °C, 5 h, 9%; (e) THF, L-Selectride (1.2 equiv), -78 °C, 4.5 h, 73%; (f) MeOH, H2O2 (3.0 equiv), 6 N NaOH (0.5 equiv), 10 °C, 2.5 h, 87%; (g) TiCl4 (1.1 equiv), THF, -78 °C; then LiCl (1.1 equiv), -20 °C; then 53 (1 equiv), -78 to -20 °C, 6 h, 89%; (h) KHMDS (1.0 equiv), DME, -78 °C, 25 min; then Ac2O, -20 °C, 54%; (i) NaOAc (2.2 equiv), HONH3Cl (1.1 equiv), MeOH, 0 °C, 100 min, 68%; THF = tetrahydrofuran, DCM = dichloromethane, KHMDS = potassium hexamethyldisilazide, DME = dimethoxyethane.
Repeated failure to convert carvone into the desired chloroketone via any sort of vicinal difunctionalization necessitated yet another strategic reevaluation. Therefore, carvone oxide (53) was examined as a starting material for the production of chloroketone 46. It was reasoned that vinylmagnesium bromide addition into the carbonyl group of the α,β-epoxyketone, followed by a semipinacol rearrangement, could allow installation of the quaternary stereocenter, contingent upon β-face attack of the organometallic reagent. Unfortunately, such conformationally constrained systems are known to favor axial (i.e., α-face) attack of nucleophiles,51 thus sterically precluding a successful semipinacol rearrangement. However, if the ring were less conformationally constrained, a nucleophile might favor equatorial approach, especially if the α-carbon could synergistically shield the α-face. As such, chlorohydrin 55 met these criteria and was prepared from carvone oxide in good yield. Unfortunately, 55 was unstable, even upon storage in an inert atmosphere, and failed to provide any desired product when treated with a variety of nucleophiles. Attempts were also made to trap the alcohol with an appropriate leaving group (mesyl or tosyl groups) before addition of the nucleophile, but to no avail.
Investigations subsequently turned toward sequences that would install the quaternary stereocenter and perform the chlorination in separate operations. Initial attempts to install the vinyl group via dissolving metal-promoted reductive alkylation of the epoxide52 to give 54 proved fruitless, as any electrophile that could be subsequently converted into the vinyl group (e.g., TBSOCH2CH2I, oxirane) failed to participate in the reaction. Alternatively, reduction of the carbonyl group to the known carveol oxide allowed investigations into the direct chlorination of this alcohol, which would be followed by installation of the quaternary stereocenter and regeneration of the ketone. Unfortunately, such chlorinations were unsuccessful due to the instability of the resulting epoxyalcohol. In an alternative strategy, enol acetate 56 was prepared from carvone oxide in order to test an intramolecular enolate addition into the epoxide, but this also proved to be fruitless. Condensation with hydroxylamine provided oxime 57, which was seemingly poised to undergo an α-substitution reaction, as was reported by Corey and co-workers.53 Reaction of the oxime with two equivalents of a cuprate reagent would first deprotonate the oxime, which could then open the epoxide and lead to the corresponding vinylnitroso compound (see Chart 5). The second equivalent of cuprate reagent could then participate in a 1,4-addition into the vinylnitroso group, thereby installing the quaternary center. Unfortunately, divinylcuprate was unsuccessful at accomplishing this transformation.
Chart 5.

Anticipated vinylnitroso intermediate.
After several other failed attempts to generate the desired chloroketone 46, a successful route was finally developed, inspired by a reaction developed in the Wender laboratory.54 In Wender's study, α,β-epoxyketones were first treated with strong base to form the corresponding enolate. Nucleophilic addition of an organometallic reagent (usually Grignard reagents) to this epoxyenolate at the α-carbon (as opposed to the usual β-attack), formed the corresponding α-alkyl-β-hydroxyketone, and subsequent elimination of the hydroxyl group furnished the α-substituted enone. However, a quaternary carbon installation during the course of this reaction was unprecedented. Despite this potential limitation, the reaction was attempted on carvone oxide to provide 59 in about 30% yield (Scheme 10). Extensive optimization efforts (solvent, nucleophile, base, additives, temperature, addition rates) did not result in significant improvement in the overall efficiency of the reaction. The low yield is likely due to the sterically hindered nature at the α-position of the α,β-epoxyketone, causing SN2' attack (58, red arrow) to be favored over the desired SN2 attack (58, blue arrow).55 Nevertheless, with alcohol 59 readily available, the chlorination was accomplished (NCS/PPh3) in acceptable yield to provide the key chloroketone (46).56 Despite the modest overall yield of this two-step sequence, it can be used to rapidly prepare multi-gram quantities of 46 and was therefore deemed as an acceptable solution to this problem, especially given the difficulties in preparing 46 via other routes (vide supra). It is noteworthy that 59 was the only neopentyl alcohol that could be successfully chlorinated during the course of these studies. It is also interesting that varying amounts of the diastereomer (at C(13)) are generated, although these chlorination conditions often proceed with complete inversion of stereochemistry. Perhaps this alcohol is more accessible to electrophiles due to a slight bond-lengthening effect (i.e., retro-aldol ability), thus mitigating the neopentyl hindrance and allowing epimerization to a minor extent.
Scheme 10.
Total synthesis of ent-12-epi-fischerindole G. Reagents and conditions: (a) LHMDS (1.2 equiv), THF, -78 °C, 30 min; then H2C=CHMgBr, -15 °C, 15 min, 30%; (b) PPh3 (1.0 equiv), NCS (1.0 equiv), THF, 18 h, 55%; (c) indole (2.0 equiv), LHMDS (3.2 equiv), THF, -78 °C, 30 min; then copper(II)2-ethylhexanoate (1.5 equiv), -78 to 23 °C, 20 min, 62%; (d) Montmorillonite K-10 (10 wt. equiv), DME, microwave, 120 °C, 6 min, 26% isolated, 40% isolated after one recycling, 57% brsm; (e) NaBH4 (1.5 equiv), MeOH, 0 °C, 5 min, 100%; (f) Ms2O (2.0 equiv), Pyr., 23 °C, 30 min, 69%; (g) LiN3 (3.0 equiv), DMF, 120 °C, 48 h, 58%; (h) Na(Hg) (10 equiv), EtOH, reflux, 4 h, 66%; (i) HCO2H (1.3 equiv), CDMT (1.4 equiv), DMAP (0.03 equiv), NMM (1.4 equiv), DCM, 23 °C, 30 min, 87%; (j) Burgess reagent (2.0 equiv), PhH, 23 °C, 30 min, 82%; LHMDS = lithium hexamethyldisilazide, THF = tetrahydrofuran, NCS = N-chlorosuccinimide, DME = dimethoxyethane, Pyr. = pyridine, DMF = dimethylformamide, EtOH = ethanol, CDMT = 2-chloro-4,6-dimethoxy-1,3,5-triazine, DMAP = 4-(dimethylamino)pyridine, NMM = N-methylmorpholine, DCM = dichloromethane, DMT-MM = 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride, PhH = benzene.
With 46 in hand, the stage was finally set to invoke the direct indole coupling reaction, which proceeded smoothly to provide the coupled product (45) in 62% yield as a single diastereomer (verified by X-ray crystallographic analysis). Several aspects of this particular coupling are noteworthy. First, it is remarkable that chloride elimination is not observed during the course of this coupling. Second, it was discovered that as the reaction concentration was increased, the yield improved. Third, any C(13) diastereomer of 46 present in the reaction mixture does not participate in the coupling reaction and rather suffers elimination, presumably due to the axial disposition of the chlorine atom. Finally, this effective method for direct C-C bond formation enables all the necessary carbon atoms of these complex natural products to be secured in only three steps and was routinely carried out on multi-gram scale. Functional group manipulations are all that remained to complete the synthesis of 12.
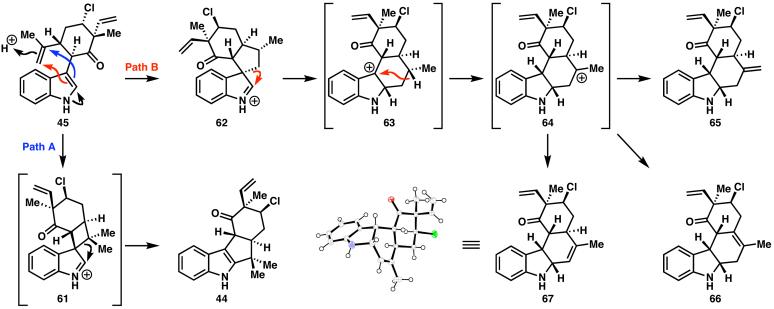
Conditions (i.e., TfOH) previously applied to the conversion of 41 to 43 unfortunately provided significant quantities of several byproducts in the attempted conversion of 45 to 44,57 including 65, 66, and 67 (Scheme 11). In this particular cyclization reaction, two modes of activation are feasible at the gem-disubstituted olefin. This olefin can be protonated to give the tertiary carbocation, which is intercepted by the indole ring to give intermediate 61, which in turn leads to the desired product (44). Alternatively, protonation to give the primary carbocation, which is also intercepted by the indole ring, leads to intermediate 62. A [1,5]-sigmatropic shift generates 63, which can then rearrange to carbocation 64. The three possible modes of elimination to quench this carbocation provide the three observed products (65, 66, and 67; 67: clear cubes, mp 128-129 °C, see Scheme 11 for X-ray crystallographic analysis). In order to circumvent the formation of such undesired byproducts, a variety of Lewis and Brønsted acids were screened (TFA, HCl, MeOSO2H, H2SO4, heat, TsOH, BF3•Et2O, AcOH, Tf2NH, Dy(OTf)3, PPTS, AlCl3, FeCl3, silicotungstic acid, PtCl2, zeolite NaY, RuCl3/AgOTf, Cu(OTf)2, Pd(OAc)2, Co(acac)2/PhSiH3), many of which did not catalyze the cyclization and none of which provided either any improvement in the yield or reduction in the quantity of byproducts. Attempted cyclizations of the amine or alcohol derivatives of 45 were equally unsuccessful. Finally, it was found that Montmorillonite K-10 acidic clay, with microwave irradiation, provided the desired product (44) without formation of the undesired byproducts, although recycling of unreacted starting material was required. It was assumed, based on the reactivity observed for 43, that reductive amination of the ketone 44 would provide amine 60 directly. However, reductive amination under the previously employed conditions (see Scheme 7)58 provided amine 70 (see Scheme 13) as a single diastereomer, which was epimeric at C(11) (as was required for 10). This unexpected complete inversion in diastereoselectivity seems to be solely due to the presence of the C(13) chlorine atom. A more circuitous route was therefore required to access the desired amine (60), necessitating reduction to the alcohol, mesylation, azidation, and reduction (Scheme 10), similar to the sequence developed by Fukuyama and co-workers for hapalindole G.7 Formylation59 of the amine (60) followed by dehydration with Burgess reagent60 provided ent-12-epi-fischerindole G (10),16 which was spectroscopically identical to the natural material with the exception of optical rotation. Thus, in principle, the naturally occurring enantiomer of 10 could be prepared from (S)-carvone oxide (vide infra).61
Scheme 11.
Formation of the acid-catalyzed cyclization byproducts.
Scheme 13.
Original and improved total synthesis of 12-epi-fischerindole I (21). Reagents and conditions: (a) NH4OAc (40 equiv), NaCNBH3 (7.5 equiv), 3 Å molecular sieves, MeOH, THF, sonication, 18 h, 42%; (b) HCO2H (2.0 equiv), CDMT (2.2 equiv), DMAP (0.1 equiv), NMM (2.2 equiv), DCM, 23 °C, 30 min, 100 %; (c) THF, TEA (1.0 equiv), t-BuOCl (1.5 equiv), 0 °C, 10 min, then SiO2/TEA (PTLC); then CDCl3, then Burgess reagent (2.0 equiv), PhH, 23 °C, 30 min, 46% overall; (d) TEA (17.5 equiv), DCM, COCl2 (2.0 equiv), 0 °C, 10 min, 95%; (e) DDQ (2.5 equiv), H2O, THF, 0 °C, 30 min, 92%; THF = tetrahydrofuran, CDMT = 2-chloro-4,6-dimethoxy-1,3,5-triazine, DMAP = 4-(dimethylamino)pyridine, NMM = N-methylmorpholine, DCM = dichloromethane, DMT-MM = 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride, TEA = triethylamine, PTLC = preparative thin layer chromatography, PhH = benzene, DDQ = 2,3-dichloro-5,6-dicyanobenzoquinone.
Total Synthesis of Fischerindole I
With reasonable quantities of 10 available, the oxidation to form 12-epi-fischerindole I (21) could be investigated. Treatment of 10 with a variety of oxidants (e.g., DDQ, MnO2, p-chloranil, t-BuOCl) failed to produce any 21 (Scheme 12). Reasoning that the isonitrile could preferentially react with these oxidants, the formamide derivative (68) was subjected to various oxidants,62 but this also failed to form any of the desired product. Putative formation of stable 3-chloro- or 3-hydroxyindolenines was the only reactivity observed with either 10 or 68, but these could not be utilized to install the required element of unsaturation. Turning to the amine diastereomer 70 (Scheme 13),63 which was ineffective for the preparation of 10, it was hoped that this intermediate would allow the desired oxidation to proceed through subtle stereoelectronic differences. Amine 70 was formylated in quantitative yield to give 71, which was then subjected to various oxidation conditions.64 Remarkably, 21 was produced in 46% overall yield when formamide 71 was treated first with t-BuOCl and triethylamine, and subsequently with Burgess reagent. Although the intermediates in this reaction are unstable and difficult to purify, it is reasonable to assume that the reaction proceeds via the intermediates delineated in Scheme 13. Initial chlorination of the indole leads to the chloroindolenine 72, which undergoes elimination to generate methylene indolenine 73. This intermediate can tautomerize to 12-epi-fischerindole I formamide (69), which is unstable and immediately dehydrates upon exposure to Burgess reagent to provide 21.16 Methylene indolenine 73 can alternatively be hydrated (presumably on silica gel) at the imine carbon. Reprotonation of the olefin from the β-face and attack by water at C(3), via the intermediacy of an azaorthoquinodimethane intermediate, provides the major side product (74, confirmed by X-ray crystallographic analysis). It is to be noted that the presence of the epimerized C(10) center provides evidence for the existence of 73 en route to 21.
Scheme 12.
Attempted conversion of ent-12-epi-fischerindole G (10) into ent-12-epi-fischerindole I (21).
Although this sequence provided the first synthetic sample of 21, it was plagued with numerous problems, specifically: Low overall yields, difficult and unscaleable synthetic procedures, the formation of byproducts, and the intermediacy of several unstable intermediates. It was reasoned that the low overall efficiency of this route likely stemmed from two controlling factors. First, t-BuOCl is not commonly employed as an oxidant for benzylic oxidations. Perhaps an oxidant more chemoselective for such transformations would provide a higher yield of the desired product. Second, the penultimate intermediate (69, Figure 1) in this sequence is an extremely unstable compound due to a severe steric interaction between the formamide N-H and the C(4)-H of the indole ring, leading to an approximate 25 kcal/mol calculated destabilization compared to its olefin-translocated isomer (76).65 Therefore, an alternate sequence was sought to accomplish this transformation while bypassing these difficulties. It was subsequently discovered that initial dehydration of formamide 71 (Scheme 13) provided isonitrile 75, which is epimeric at C(11) to 12-epi-fischerindole G (10), in 95% yield. Treatment of this compound with DDQ,66 in contrast to the DDQ reaction for 10, provided 12-epi-fischerindole I (21) in 92% yield, allowing access to large quantities of this natural product in short order.14 Based on these results, although it is quite possible that 10 could be enzymatically promoted to 21, it is equally possible that 75 (not yet isolated as a natural product), rather than 10, could be the actual biosynthetic precursor to 21.
Figure 1.
Calculated stability of the 12-epi-fischerindole I penultimate intermediate.
Total Synthesis of Welwitindolinone A
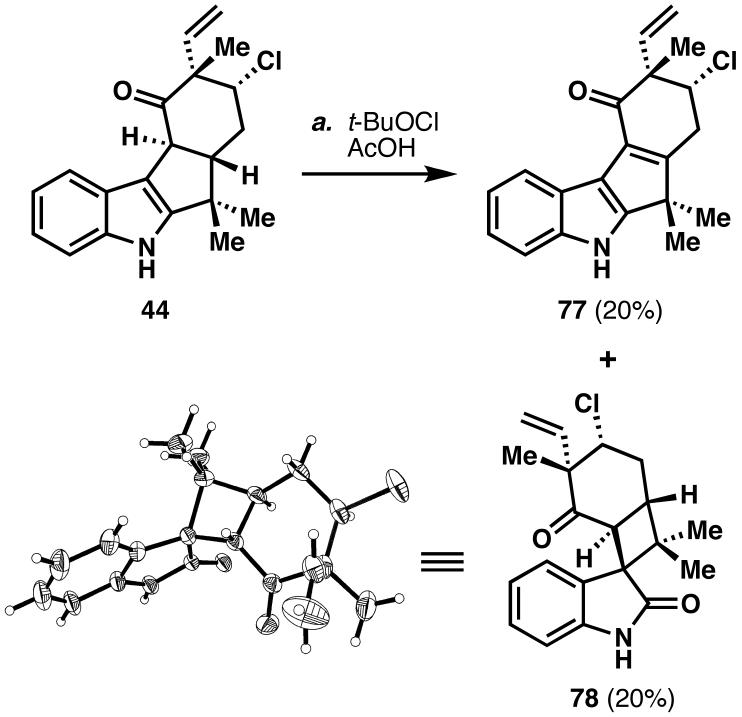
With 12-epi-fischerindole I (21) in hand, it was finally possible to investigate the conversion of this natural product into welwitindolinone A (12). Although the proposed conversion of 21 to 12 might appear intuitive, practical difficulties were expected due to the sensitivity of both alkaloids to acidic media and the sheer ring strain of the resulting product. Such concerns were intensified by the knowledge that oxidative ring contractions to generate five- and six-membered rings typically require elevated temperatures, and hence, forming a strained four-membered ring should be even more difficult.37 Before using valuable material to probe this transformation, a model system was sought on which to test this ring contraction. To our delight, treatment of cyclized ketone 44 with t-BuOCl then dilute AcOH furnished two major products: The oxidized compound 77 and ring-contracted compound 78 in approximately 20% unoptimized yield each. Given the aforementioned considerations, it is remarkable that this reaction occurs at low temperature and within minutes. Although ketone 78 represents a potentially viable intermediate to complete the synthesis of 12,67 attention was returned to 21, in the hope that it could be directly converted into 12, thus lending credence to the proposed biosynthetic hypothesis (vide supra).
Extensive experimentation led to conditions by which 21 could be converted directly into 12. 12-epi-Fischerindole I (21) was exposed to t-BuOCl and triethylamine in THF at -30 °C for one minute and the solvent was rapidly removed (Scheme 15). The crude residue was then dissolved in a THF:H2O:TFA mixture (95:4:1) and warmed to 0 °C. Strict adherence to this protocol resulted in the first total synthesis of 12 in 25% yield,16 along with minor amounts of its C(3) epimer (see Chart 6). This transformation most likely proceeds through initial chlorination of the indole to provide 79, followed by attack of water to give 80. Elimination of the chloride, to generate azaorthoquinodimethane 81, followed by [1,5]-sigmatropic rearrangement, would then install the spirocyclobutane of 12. Although 12 was accessible via this procedure, there were several problems with this route, most notably the low yield and the exclusive formation of the C(3) diastereomer upon scale-up. Seeking to circumvent these obstacles, it was determined that the chief difficulty arose from the isonitrile moiety, which has been shown to be unstable to electrophilic chlorinating reagents in this laboratory.14 It was reasoned that a hitherto-unknown fluorohydroxylation of indole rather than chlorohydroxylation should suppress isonitrile-derived byproduct formation, owing to the increased hardness of fluorine over chlorine. Therefore, a milder method to accomplish this transformation was developed, in which 21 was treated with a solution of XeF2 in wet acetonitrile to provide 12 in 44% yield, via the intermediacy of 82.14 This reaction was routinely performed on more than 50 mg of 21, and more than 580 mg of 12 have been prepared to date. A screen of various halogenating reagents confirmed that XeF2 was the most efficient at promoting this reaction (Table 2). The chemoselectivity of this reagent is also noteworthy, given the presence of an olefin, which is known to react with XeF2,68 and the reactive isonitrile moiety.
Scheme 15.
Original and improved total syntheses of welwitindolinone A (12). Reagents and conditions: (a) THF, TEA (1.0 equiv), t-BuOCl (1.5 equiv), - 30 °C, 1 min; then 95:4:1 THF:H2O:TFA, -30 to 0 °C, 5 min, 25%; (b) XeF2 (1.0 equiv), H2O, MeCN, 23 °C, 5 min, 44%; THF = tetrahydrofuran, TEA = triethylamine, TFA = trifluoroacetic acid, MeCN = acetonitrile.
Chart 6.

3-epi-Welwitindolinone A.
Table 2.
Ring contraction optimization.
| Oxidant | Yield |
|---|---|
| t-BuOCl | 0-25% |
| NBS | 0% |
| Synfluor | 0% |
| Selectfluor | 0% |
| BMAST | 0% |
| NFSI | 16% |
| XeF2 | 44% |
In addition to 21 and 12 (vide infra), the isothiocyanate analogues can be readily synthesized via the sequence described herein (Scheme 16). Treatment of amine 70 with CS(imid)2 provides isothiocyanate 83, which can be oxidized to the 12-epi-fischerindole I analogue 84 (colorless cubes, mp 202 °C decomposition, see Scheme 16 for X-ray crystallographic analysis) in 76% yield. Treatment of 84 with XeF2 provides the isothiocyanate analogue of welwitindolinone A (85) in an unoptimized 27% yield. Furthermore, the isocyanate analogue of welwitindolinone A (86) is directly available from welwitindolinone A (12) upon exposure to TFDO69 (Scheme 17). However, this particular analogue exhibited greater instability than the isonitrile and isothiocyanate derivatives and dimerized to the urea dimer 87 upon storage in air at room temperature. Despite this reactivity, it is conceivable that such intermediates might be useful for the preparation of further members of the welwitindolinone alkaloid family.
Scheme 16.
Synthesis of isothiocyanate derivatives. Reagents and conditions: (a) DCM, CS(imid)2 (3.3 equiv), 23 °C, 24 h, 60%; (b) THF, H2O, DDQ (2.5 equiv), 0 °C, 30 min, 68%; (c) DCM, XeF2 (1.0 equiv), 23 °C, 15 min, 27%; DCM = dichloromethane, THF = tetrahydrofuran, DDQ = 2,3-dichloro-5,6-dicyanobenzoquinone.
Scheme 17.
Synthesis and degradation of 86. Reagents and conditions: (a) DCM, TFDO (1.0 equiv), -78 °C, 5 min, 100%; (b) air, 23 °C, 6 d; DCM = dichloromethane, TFDO = methyl(trifluoromethyl)-dioxirane.
Oxidation Attempts Toward Welwitindolinone B
In order to test the biosynthetic hypothesis delineated in Scheme 2, and in an attempt to generate the welwitindolinone B core structure, welwitindolinone A (12) was subjected to a variety of epoxidation and general oxidation conditions. With the exception of TFDO that generated the corresponding isocyanate 86 (vide supra), all attempted conditions either led to recovered starting material or decomposition (see Supporting Information for a list of failed conditions). Consequently, three-step alternatives were envisioned, with the hope that the electronics of the tetrasubstituted olefin would be modified in a subtle fashion as to permit oxidative rearrangement (see Scheme 18). Although not ideal, transformation of the isonitrile moiety into a gem-dibromide (such as 88) or a formamide (such as 90), oxidative rearrangement into the corresponding welwitindolinone B framework (89 or 91, respectively) followed by restoration of the original isonitrile group could enable the formation of “welwitindolinone B isonitrile” (14a). With this strategy in mind, gem-dibromide 88 and formamide 90 were prepared from welwitindolinone A using phenyltrimethylammonium tribromide and formic acid, respectively, both in quantitative yields; functional group restoration to the original isonitrile 12 was also verified by using triethyl phosphite and phosgene, respectively. Unfortunately, all oxidation conditions experimented upon 88 or 90 either resulted in recovered starting material, welwitindolinone A (only when using 88), or decomposition (see Supporting Information). Since some welwitindolinones are methylated at the indole nitrogen atom (see Chart 1), N-methylformamide 92 was prepared from 12 in an attempt to alter reactivity, but this substrate also failed to undergo oxidative rearrangement (see Supporting Information).
Scheme 18.
Oxidative rearrangement attempts. Reagents and conditions: (a) PTAT (1 equiv), DCM, -78 °C, 3 min, 100%; (b) P(OEt)3, DCM, 23 °C, 3 min, 100%; (c) HCO2H, H2O, THF, 4 °C, 12 h, 100%; (d) TEA, COCl2, DCM, 0° C, 10 min, 82%; (e) MeI, K2CO3, acetone, 23 °C, 12 h, 100%; (f) HCO2H, H2O, THF, 4 °C, 12 h, 100%; PTAT = phenyltrimethylammonium tribromide, DCM = dichloromethane, THF = tetrahydrofuran, TEA = triethylamine.
These preliminary results do not necessarily contradict the biosynthetic hypothesis toward welwitindolinone B put forth in Scheme 2; perhaps the oxidation requisite for this transformation is enzymatically controlled.
Strategy Analysis and Conclusions
Due to their stunning molecular architectures and potent bioactivities, the hapalindoles, fischerindoles, and welwitindolinones were targeted for total synthesis, with the goals of inventing useful chemistry, discovering basic reactivity, understanding their biosynthesis, and allowing access to large quantities of these rare marine natural products. It is instructive to evaluate these syntheses from the vantage points of chemoselectivity, stereocontrol, and “redox economy”.
Numerous steps throughout this synthesis exhibited high levels of chemoselectivity, despite the presence of other reactive functionality. For example, the conversion of 46 to 45 proceeds in good yield, in the presence of a chlorine atom that could potentially be eliminated under the reaction conditions. The vast majority of conditions screened for the cyclization of 45 to 44 formed numerous byproducts (including 65, 66, and 67); however, the use of Montmorillonite K-10 acidic clay completely avoided such chemical entities. The conversion of 75 into 21 proceeds in excellent yield, despite the ease with which isonitriles can be oxidized, and the conversion of 21 to 12 proceeds in good yield, even though isonitriles have been observed to react with halogenating reagents.14 Functional group manipulations were minimized and the percentage of C-C bond-forming reactions was maximized. It is difficult to imagine how any steps could be “removed” from these syntheses, since all are necessary for the installation of requisite C-C bonds, functional groups, or key stereocenters. Furthermore, no protecting groups are utilized throughout the course of this entire synthesis, despite numerous opportunities in which they could potentially have been employed to circumvent undesirable reactivity. In fact, rather than resorting to protecting group chemistry, the innate reactivities of the functional groups were employed, which led to the invention of new chemistry (direct indole coupling, extremely mild fluorohydroxylative ring-contraction using XeF2, installation of the key quaternary stereocenter and neopentyl chlorine atom) or discovery of intriguing reactivity (49 to 50, 45 to 65, 66, and 67, 71 to 21 and 74, 75 to 21, 44 to 78, and 21 to 12).
High levels of stereochemical induction are observed throughout the synthesis. For example, the direct indole coupling reaction (31 and 45) provides a single diastereomer of coupled product and the reductive alkylation of 31 to provide 41 only gives a single diastereomer. The reductive aminations proceed in moderate (41 to 27, which could be increased with the use of microwave irradiation) to complete diastereoselectivity (43 to 29 and 44 to 70). Furthermore, by utilizing XeF2, complete diastereomeric induction is observed in the conversion of 21 to 12, even though the facial bias for this transformation is minimal.
Finally, the syntheses are characterized by an adherence to the concept of “redox economy”. Analogous to “atom economy”70 or “step economy”,71 “redox economy”72 minimizes the superfluous redox manipulations within a synthesis; rather, the oxidation state of intermediates linearly and steadily increases throughout the course of the synthesis. Only one reduction step (reductive amination) is utilized during these syntheses, which is strategically placed to install a key stereocenter. In fact, the flexible adherence of these syntheses to the proposed biogenesis of these alkaloids reinforces the minimization of redox reactions, as is commonly observed in Nature's biosynthesis of terpenes and alkaloids. Such considerations allowed the efficient, practical, and concise syntheses of numerous members of this natural product family (10, 12, 21, 27, 29, and 42). Overall, this total synthesis program is characterized by inventive retrosynthetic disconnections, which rapidly assemble the skeletal structure and allow for rapid increase in complexity. These studies are yet another example of how natural products can catalyze new discoveries in chemical reactivity.
Supplementary Material
Scheme 14.
Model system for the total synthesis of welwitindolinone A (12). Reagents and conditions: (a) THF, TEA (1.0 equiv), t-BuOCl (1.7 equiv), 0 °C, 10 min; then 40:20:1 MeOH:H2O:AcOH, 5 min; THF = tetrahydrofuran, TEA = triethylamine.
ACKNOWLEDGMENT
We thank Dr. D. H. Huang and Dr. L. Pasternack for NMR spectroscopic assistance, and Dr. G. Siuzdak for mass spectrometric and Dr. A. Rheingold and Dr. R. Chadha for X-ray crystallographic assistance. We are grateful to the National Science Foundation and Bristol-Myers Squibb for predoctoral fellowships (J. M. R.), The Scripps Research Institute for a predoctoral fellowship (Y. I.), Daiichi-Sankyo Co. for a postdoctoral fellowship (T. M.), Helsinki University of Technology for a predoctoral fellowship (A. P.), and Universidad Autónoma de Madrid for a predoctoral fellowship (T. L.). Financial support for this work was provided by The Scripps Research Institute, Amgen, AstraZeneca, the Beckman Foundation, Bristol-Myers Squibb, DuPont, Eli Lilly, GlaxoSmithKline, Pfizer, Roche, the Searle Scholarship Fund, the Sloan Foundation, and the NIH (NIGMS).
SUPPORTING INFORMATION PARAGRAPH Full characterization, including copies of 1H and 13C NMR spectra, and experimental procedures for selected compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Moore RE, Cheuk C, Patterson GML. J. Am. Chem. Soc. 1984;106:6456–6457. [Google Scholar]
- 2.(a) Moore RE, Cheuk C, Yang X-QG, Patterson GML, Bonjouklian R, Smitka TA, Mynderse JS, Foter RS, Jones ND, Swartzendruber JK, Deeter JB. J. Org. Chem. 1987;52:1036–1043. [Google Scholar]; (b) Schwartz RE, Hirsch CF, Springer JP, Pettibone DJ, Zink DL. J. Org. Chem. 1987;52:3704–3706. [Google Scholar]; (c) Moore RE, Yang X-QG, Patterson GML. J. Org. Chem. 1987;52:3773–3777. [Google Scholar]; (d) Smitka TA, Bonjouklian R, Doolin L, Jones ND, Deeter JB, Yoshida WY, Prinsep MR, Moore RE, Patterson GML. J. Org. Chem. 1992;57:857–861. [Google Scholar]; (e) Park A, Moore RE, Patterson GML. Tetrahedron Lett. 1992;33:3257–3260. [Google Scholar]; (f) Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J. Am. Chem. Soc. 1994;116:9935–9942. [Google Scholar]; (g) Huber U, Moore RE, Patterson GML. J. Nat. Prod. 1998;61:1304–1306. doi: 10.1021/np9801561. [DOI] [PubMed] [Google Scholar]; (h) Jimenez JI, Huber U, Moore RE, Patterson GML. J. Nat. Prod. 1999;62:569–572. doi: 10.1021/np980485t. [DOI] [PubMed] [Google Scholar]; (i) Klein D, Daloze D, Braekman JC, Hoffmann L, Demoulin V. J. Nat. Prod. 1995;58:1781–1785. doi: 10.1021/np9900324. [DOI] [PubMed] [Google Scholar]; (j) Moore RE, Yang X-QG, Patterson GML, Bonjouklian R, Smitka TA. Phytochemistry. 1989;28:1565–1567. [Google Scholar]; (k) Raveh A, Carmeli S. J. Nat. Prod. 2007;70:196–201. doi: 10.1021/np060495r. [DOI] [PubMed] [Google Scholar]; (l) Becher PG, Keller S, Jung G, Süssmuth RD, Jüttner F. Phytochemistry. 2007;68:2493–2497. doi: 10.1016/j.phytochem.2007.06.024. [DOI] [PubMed] [Google Scholar]; (m) Asthana RK, Srivastava A, Singh AP, Deepali, Singh SP, Nath G, Srivastava R, Srivastava BS. J. Appl. Phycol. 2006;18:33–39. [Google Scholar]
- 3.These locations include the Marshall Islands (ref. 1), the Everglades in Florida (ref. 2b), Australia (ref. 2f), Micronesia (ref. 2h), Papua New Guinea (ref. 2i), and Israel (ref. 2k)
- 4.(a) Doan NT, Rickards RW, Rothschild JM, Smith GD. J. Appl. Phycol. 2000;12:409–416. [Google Scholar]; (b) Doan NT, Stewart PR, Smith GD. FEMS Microbiol. Lett. 2001;196:135–139. doi: 10.1111/j.1574-6968.2001.tb10554.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith CD, Zilfou JT, Stratmann K, Patterson GML, Moore RE. Mol. Pharmacol. 1995;47:241–247. [PubMed] [Google Scholar]
- 6.Zhang X, Smith CD. Mol. Pharmacol. 1996;49:288–294. [PubMed] [Google Scholar]
- 7.Fukuyama T, Chen X. J. Am. Chem. Soc. 1994;116:3125–3126. [Google Scholar]
- 8.Muratake H, Kumagami H, Natsume M. Tetrahedron. 1990;46:6351–6360. [Google Scholar]
- 9.(a) Muratake H, Natsume M. Tetrahedron Lett. 1989;30:1815–1818. [Google Scholar]; (b) Muratake H, Natsume M. Tetrahedron. 1990;46:6331–6342. [Google Scholar]; (c) Muratake H, Natsume M. Tetrahedron. 1990;46:6343–6350. [Google Scholar]
- 10.(a) Baran PS, Richter JM. J. Am. Chem. Soc. 2004;126:7450–7451. doi: 10.1021/ja047874w. [DOI] [PubMed] [Google Scholar]; (b) Vaillancourt V, Albizati KF. J. Am. Chem. Soc. 1993;115:3499–3502. [Google Scholar]; (c) Kinsman AC, Kerr MA. Org. Lett. 2001;3:3189–3191. doi: 10.1021/ol0165138. [DOI] [PubMed] [Google Scholar]; (d) Kinsman AC, Kerr MA. J. Am. Chem. Soc. 2003;125:14120–14125. doi: 10.1021/ja036191y. [DOI] [PubMed] [Google Scholar]
- 11.Sakagami M, Muratake H, Natsume M. Chem. Pharm. Bull. 1994;42:1393–1398. [Google Scholar]
- 12.(a) Kinsman AC, Kerr MA. Org. Lett. 2000;2:3517–3520. doi: 10.1021/ol0065773. [DOI] [PubMed] [Google Scholar]; (b) Brown MA, Kerr MA. Tetrahedron Lett. 2001;42:983–985. [Google Scholar]; (c) Banwell MG, Ma X, Taylor RM, Willis AC. Org. Lett. 2006;8:4959–4961. doi: 10.1021/ol062020x. [DOI] [PubMed] [Google Scholar]
- 13.Chandra A, Viswanathan R, Johnston JN. Org. Lett. 2007;9:5027–5029. doi: 10.1021/ol702247a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404–408. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- 15.(a) Wood JL, Holubec AA, Stoltz BM, Weiss MM, Dixon JA, Doan BD, Shamji MF, Chen JM, Heffron TP. J. Am. Chem. Soc. 1999;121:6326–6327. [Google Scholar]; (b) Deng H, Konopelski JP. Org. Lett. 2001;3:3001–3004. doi: 10.1021/ol016379r. [DOI] [PubMed] [Google Scholar]; (c) Jung ME, Slowinski F. Tetrahedron Lett. 2001;42:6835–6838. [Google Scholar]; (d) López-Alvarado P, García-Granda S, Álvarez-Rúa C, Avendaño C. Eur. J. Org. Chem. 2002:1702–1707. [Google Scholar]; (e) Avendaño C, Menéndez JC. Curr. Org. Synth. 2004;1:65–82. [Google Scholar]; (f) Ready JM, Reisman SE, Hirata M, Weiss MM, Tamaki K, Ovaska TV, Wood JL. Angew. Chem. Int. Ed. 2004;43:1270–1272. doi: 10.1002/anie.200353282. [DOI] [PubMed] [Google Scholar]; (g) MacKay JA, Bishop RL, Rawal VH. Org. Lett. 2005;7:3421–3424. doi: 10.1021/ol051043t. [DOI] [PubMed] [Google Scholar]; (h) Baudoux J, Blake AJ, Simpkins NS. Org. Lett. 2005;7:4087–4089. doi: 10.1021/ol051239t. [DOI] [PubMed] [Google Scholar]; (i) Greshock TJ, Funk RL. Org. Lett. 2006;8:2643–2645. doi: 10.1021/ol0608799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Lauchli R, Shea KJ. Org. Lett. 2006;8:5287–5289. doi: 10.1021/ol0620747. [DOI] [PubMed] [Google Scholar]
- 16.Baran PS, Richter JM. J. Am. Chem. Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r. [DOI] [PubMed] [Google Scholar]
- 17.(a) Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J. Am. Chem. Soc. 2006;128:1448–1449. doi: 10.1021/ja057640s. [DOI] [PubMed] [Google Scholar]; (b) Reisman SE, Ready JM, Weiss MM, Hasuoka A, Hirata M, Tamaki K, Ovaska TV, Smith CJ, Wood JL. J. Am. Chem. Soc. 2008;130:2087–2100. doi: 10.1021/ja076663z. [DOI] [PubMed] [Google Scholar]
- 18.See the following texts, and references therein: Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. Wiley-VCH; Weinheim: 1996. . Nicolaou KC, Snyder SA. Classics in Total Synthesis II. Wiley-VCH; Weinheim: 2003.
- 19.Ugi I. Isonitrile Chemistry. Academic Press; New York, NY: 1971. [Google Scholar]
- 20.(a) Baran PS, Shenvi RA, Mitsos CA. Angew. Chem. Int. Ed. 2005;44:3714–3717. doi: 10.1002/anie.200500522. [DOI] [PubMed] [Google Scholar]; (b) Baran PS, Shenvi RA. J. Am. Chem. Soc. 2006;128:14028–14029. doi: 10.1021/ja0659673. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs JR, Funk RL. Org. Lett. 2005;7:677–680. doi: 10.1021/ol047532v. [DOI] [PubMed] [Google Scholar]
- 22.Kovács-Kulyassa Á, Herczegh P, Sztaricskai F. Tetrahedron. 1997;53:13883–13896.. Also see: Barton DHR, Crich D. J. Chem. Soc. Chem. Commun. 1984:774–775.
- 23.Nicolaou KC, Vassilikogiannakis G, Kranich R, Baran PS, Zhong Y-L, Natarajan S. Org. Lett. 2000;2:1895–1898. doi: 10.1021/ol000102u.. Also see: Barton DHR, McCombie SW. J. Chem. Soc. Perkin Trans. 1. 1975:1574–1585.
- 24.retro-Aldol reaction was the major competing pathway
- 25.Martin JC, Arhart RJ. J. Am. Chem. Soc. 1971;93:4327–4329. [Google Scholar]
- 26.Burgess EM, Penton HR, Jr., Taylor EA. J. Org. Chem. 1973;38:26–31. [Google Scholar]
- 27.Nicolaou KC, Hao J, Reddy MV, Rao PB, Rassias G, Snyder SA, Huang X, Chen DY-K, Brenzovich WE, Giuseppone N, Giannakakou P, O'Brate A. J. Am. Chem. Soc. 2004;126:12897–12906. doi: 10.1021/ja040093a. [DOI] [PubMed] [Google Scholar]
- 28.(a) Weinberg NL, Weinberg HR. Chem. Rev. 1968;68:449–523. [Google Scholar]; (b) Kauffmann T. Angew. Chem. Int. Ed. 1974;13:291–305. [Google Scholar]; (c) Csákÿ AG, Plumet J. Chem. Soc. Rev. 2001;30:313–320. [Google Scholar]; (d) Baran PS, Ambhaikar NA, Guerrero CA, Hafensteiner BD, Lin DW, Richter JM. ARKIVOC. 2006;vii:310–325. [Google Scholar]
- 29.Richter JM, Whitefield BW, Maimone TJ, Lin DW, Castroviejo MP, Baran PS. J. Am. Chem. Soc. 2007;129:12857–12869. doi: 10.1021/ja074392m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.(a) Fang C-L, Horne S, Taylor N, Rodrigo R. J. Am. Chem. Soc. 1994;116:9480–9486. [Google Scholar]; (b) Ito Y, Konoike T, Saegusa T. J. Am. Chem. Soc. 1975;97:2912–2914. [Google Scholar]; (c) Ito Y, Konoike T, Harada T, Saegusa T. J. Am. Chem. Soc. 1977;99:1487–1493. [Google Scholar]
- 31.Wang J-J, Hu W-P. J. Org. Chem. 1999;64:5725–5727. doi: 10.1021/jo990549k. [DOI] [PubMed] [Google Scholar]
- 32.(a) Baran PS, DeMartino MP. Angew. Chem. Int. Ed. 2006;45:7083–7086. doi: 10.1002/anie.200603024. [DOI] [PubMed] [Google Scholar]; (b) DeMartino MP, Chen K, Baran PS. J. Am. Chem. Soc. 2008;130:11546–11560. doi: 10.1021/ja804159y. [DOI] [PubMed] [Google Scholar]
- 33.Barton DHR, Deflorin AM, Edwards OE. J. Chem. Soc. 1956:530–534. [Google Scholar]
- 34.Baran PS, Richter JM, Lin DW. Angew. Chem. Int. Ed. 2005;44:609–612. doi: 10.1002/anie.200462048. [DOI] [PubMed] [Google Scholar]
- 35.Fortunato JM, Ganem B. J. Org. Chem. 1976;41:2194–2200. [Google Scholar]
- 36.Prolonged exposure to triflic acid led to product decomposition; therefore, the reaction was quenched early and the starting material was recycled
- 37.Witkop B, Patrick JB. J. Am. Chem. Soc. 1953;75:2572–2576. [Google Scholar]
- 38.(a) Dhanak D, Neidle S, Reese CB. Tetrahedron Lett. 1985;26:2017–2020. [Google Scholar]; (b) Lee BH, Clothier MF, Johnson SS. Bioorg. Med. Chem. Lett. 2001;11:553–554. doi: 10.1016/s0960-894x(00)00698-3. [DOI] [PubMed] [Google Scholar]; (c) Williams RM, Cao J, Tsujishima H, Cox RJ. J. Am. Chem. Soc. 2003;125:12172–12178. doi: 10.1021/ja036713+. [DOI] [PubMed] [Google Scholar]
- 39.Wenkert E, Moeller PDR, Piettre SR, McPhail AT. J. Org. Chem. 1987;52:3404–3409. [Google Scholar]
- 40.(a) Gorthey LA, Vairamani M, Djerassi C. J. Org. Chem. 1985;50:4173–4182. [Google Scholar]; (b) Albone KS, Macmillan J, Pitt AR, Willis CL. Tetrahedron. 1986;42:3203–3214. [Google Scholar]
- 41.Li G, Gao J, Wei H-X, Enright M. Org. Lett. 2000;2:617–620. doi: 10.1021/ol9904040. [DOI] [PubMed] [Google Scholar]
- 42.Carlsen PHJ, Katsuki T, Martin VS, Sharpless KB. J. Org. Chem. 1981;46:3936–3938. [Google Scholar]
- 43.Lee CA, Floreancig PE. Tetrahedron Lett. 2004;45:7193–7196. [Google Scholar]
- 44.Corey EJ, Jautelat M. Tetrahedron Lett. 1968;9:5787–5788. [Google Scholar]
- 45.Onishi Y, Ogawa D, Yasuda M, Baba A. J. Am. Chem. Soc. 2002;124:13690–13691. doi: 10.1021/ja0283246. [DOI] [PubMed] [Google Scholar]
- 46.Haufe G, Wolf A, Schulze K. Tetrahedron. 1986;42:4719–4728. [Google Scholar]
- 47.De Luca L, Giacomelli G, Porcheddu A. Org. Lett. 2002;4:553–555. doi: 10.1021/ol017168p. [DOI] [PubMed] [Google Scholar]
- 48.Nicolaou KC, Mathison CJN, Montagnon T. J. Am. Chem. Soc. 2004;126:5192–5201. doi: 10.1021/ja0400382. [DOI] [PubMed] [Google Scholar]
- 49.Iwama T, Matsumoto H, Shimizu H, Kataoka T, Muraoka O, Tanabe G. J. Chem. Soc. Perkin Trans. 1. 1998:1569–1576. [Google Scholar]
- 50.Kreh DW, Krug RC. J. Org. Chem. 1967;32:4057–4059. [Google Scholar]
- 51.(a) Chérest M, Felkin H. Tetrahedron Lett. 1968;9:2205–2208. [Google Scholar]; (b) Huet J, Maroni-Barnaud Y, Anh NT, Seyden-Penne J. Tetrahedron Lett. 1976;17:159–162. [Google Scholar]; (c) Stork G, Stryker JM. Tetrahedron Lett. 1983;24:4887–4890. [Google Scholar]; (d) Gung BW. Chem. Rev. 1999;99:1377–1386. doi: 10.1021/cr980365q. [DOI] [PubMed] [Google Scholar]
- 52.Caine D, McCloskey CJ, Van Derveer D. J. Org. Chem. 1985;50:175–179. [Google Scholar]
- 53.Corey EJ, Melvin LS, Jr., Haslanger MF. Tetrahedron Lett. 1975;16:3117–3120. [Google Scholar]
- 54.Wender PA, Erhardt JM, Letendre LJ. J. Am. Chem. Soc. 1981;103:2114–2116. [Google Scholar]
- 55.This alternate reaction pathway was also noted in Wender's studies
- 56.Alternative chlorination conditions were attempted (SOCl2/base, PPh3/CCl4, PPh3/ZnCl2/DEAD, MsCl/Pyr., PPh3/Cl2, DMAP/CS(imid)2, (COCl)2, TCT, TMSCl/BiCl3) but were unsuccessful at providing the desired product, and retro-aldol product was observed on at least one occasion
- 57.In fact, the reactivity of 47 and downstream intermediates differed significantly from that observed with the 12-epi-fischerindole U series
- 58.Attempts to utilize microwave irradiation to promote this reaction led to dechlorination of the compound and was therefore not amenable for this series of chlorinated natural products
- 59.De Luca L, Giacomelli G, Porcheddu A, Salaris M. Synlett. 2004;14:2570–2572. [Google Scholar]
- 60.Creedon SM, Crowley HK, McCarthy DG. J. Chem. Soc. Perkin Trans. 1. 1998:1015–1017. [Google Scholar]
- 61.Until this point, for initial studies into the synthesis of these natural products, (R)-carvone oxide was used because of its significantly lower cost
- 62.The same oxidants were utilized as for 10, as well as DMDO and DMP
- 63.From this point on, (S)-carvone oxide was utilized to allow access to the correct enantiomer of the natural product
- 64.It was assumed that the isonitrile might react disastrously with various oxidants, therefore the formamide was utilized in these trials
- 65.As determined by AM1 calculations
- 66.Oikawa Y, Yonemitsu O. J. Org. Chem. 1977;42:1213–1216. [Google Scholar]
- 67.The Wood group recently reported that their attempts to convert 10-epi-78 into 12 were unsuccessful, utilizing a variety of conditions (see ref. 17b)
- 68.Shellhamer DF, Carter DL, Chiaco MC, Harris TE, Henderson RD, Low WSC, Metcalf BT, Willis MC, Heasley VL, Chapman RD. J. Chem. Soc. Perkin Trans 2. 1991:401–403. [Google Scholar]
- 69.Mello R, Fiorentino M, Fusco C, Curci R. J. Am. Chem. Soc. 1989;111:6749–6757. [Google Scholar]
- 70.Trost BM. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 71.Wender PA, Miller BL. In: Organic Synthesis: Theory and Applications. Hudlicky T, editor. Vol. 2. JAI Press; Greenwich, CT: 1993. [Google Scholar]
- 72.For illuminating discussions of oxidation state control in synthesis, see: Evans DA, Andrews GC. Acc. Chem. Res. 1974;7:147–155.; Hendrickson JB. J. Am. Chem. Soc. 1975;97:5784–5800.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.