Abstract
The growth and circumnutation of the stem of three-week old Helianthus annuus in the 16:8 h light:dark photoperiod were monitored using an angular position-sensing transducer and a time lapse photography system. It was found that the rate of growth and circumnutation reached a high level in the dark stage; in the light stage, however, only the growth rate reached the same high level, whereas the circumnutations were weak. These results showed that in the light stage the stem circumnutation was downregulated more strongly than the growth. Short-term stem responses to darkening and illumination were a further display of the relation between growth and circumnutations. Switching off the light caused an increase in the growth and circumnutation rate. In some cases it was accompanied by changes in the rotation direction. On the other hand, switching the light on caused an immediate transient (several-minute long) decrease in the growth rate resulting in stem contraction, and this was accompanied by an almost complete pause of circumnutation. Additionally, under light, there occurred a subsequent decrease in the magnitude, disturbance of circumnutation trajectory and, in some cases, changes in the direction of rotation. The observed stem contraction and disturbance of circumnutation imply the occurrence of turgor changes in sunflower stem, which may be caused by a non-wounding, darkening or illumination stimulus. Our experiments indicate that the disturbances of the growth rate are accompanied by changes in circumnutation parameters but we have also seen that there is no simple quantitative relation between growth rate and circumnutation rate.
Key words: Helianthus annuus, plant movement, circumnutation, elongation, growth, stem contraction
Introduction
The circumnutation phenomenon, widely spread among plants, is defined as endogenous, helical movement of growing organs. It is emphasized that organ elongation is essential for the occurrence of circumnutations, which are merely a means and consequence of plant organ elongation.1,2 In plants, the growth rate may fluctuate in a short-time scale in the range of minutes to hours, and in a large-time scale, ranging from hours to days.3–7 Daily, circadian and infradian rhythms were also observed in the changes of circumnutation intensity.8–12 It is known that helical growth may be interrupted by periods of straight growth,13 which is known as nutating and non-nutating phases of growth, for example, in Helianthus annuus seedlings.1,14 Quantitative relations between growth rate and the occurrence of circumnutations have been shown for Periploca gracea shoots, where the threshold growth rate value above which circumnutations were detectable was 0.5 mm h−1.15,16 In Arabidopsis thaliana seedlings there appeared short-period nutations (20–60 min) during the highest growth rate, while during the low growth rate there were long-period nutations (1–8 h). At a significantly reduced growth rate the hypocotyls did not circumnutate.8 These data support the existence of a threshold growth rate, below which circumnutations do not occur; they also confirm the correlation between the circadian-like fashion of stem growth and the circumnutation occurrence. There have been experiments conducted in which some chemical treatment discriminated between organ movement and organ elongation. That was done by application of lithium chloride, which inhibited circumnutation without altering growth in Phaseolus vulgaris shoots16 and in Helianthus annuus hypcotyls.17 Moreover, Hayashi and al.,18 have reported lately that aluminium ion treatment ceased the rotation movement but not the elongation of the roots in Oryza sativa. Therefore, circumnutation can be blocked without simultaneous inhibition of growth. All these facts indicate that growth may take place without circumnutation. Therefore, can this mutual independence be a result of reversible cell volume changes in the circumnutation mechanism? Experiments conducted on three-week old sunflower have shown that a wounding stimulus induces both growth disturbances of the contraction/elongation type and characteristic changes in the circumnutation trajectory.19,20 Partly reversible cell volume changes, mediated by turgor and involved in the circumnutations of Phaseolus vulgaris shoots have also been described.21 Thus, the transient reversible stem deformations (contraction) could probably accompany the circumnutations themselves and circumnutation disorders. Furthermore, a capability for light-induced transient contraction related to growth inhibition was observed in maize coleoptiles and in Arabidopsis hypocotyls.22,23 These examples indicate that in plant not only stomata and pulvini cells, but also shoot cells and hypocotyls protoplasts display the ability for transient contraction. In this study, growth rate and circumnutation changes were measured simultaneously in 16:8 h light:dark photoperiod in order to show the relationship between them. The purpose was to characterize the short- and long-term growth rate changes and the changes in circumnutation rate and trajectory after darkening and illumination. The light-induced changes in the circumnutation trajectory have not been described so far.
Results
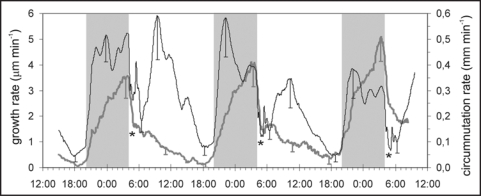
When measured at the apex of the plant, the stem elongation rate reached approximately 10 mm per day. 50 mm below the apex, in the place chosen for growth measurement, it was 5 mm per day. Insertion of the transducer needle in the stem in this place partly immobilized the upper part of the stem and reduced the length of circumnutation trajectory by about 50%, but the changes in relation to time were typical and similar to circumnutations in intact plants. The angular position-sensing transducer used for recording of the stem growth was sensitive to circumnutation movements, which were visible as ultradian changes against a diurnal background. The comparison of the diurnal fluctuations of growth rate and circumnutation rate during three days is presented in Figure 1. The growth rate reached the maximum level (3–6 µm min−1) during the dark period and before noon. At the same time, the circumnutation rate reached a high level (0.3–0.5 mm min−1) only during the dark stage, whereas in the morning it was weak (0.1 mm min−1), despite the high level of growth rate. At about 18:00 the rate of growth and circumnutations was the lowest.
Figure 1.
Comparison of growth and circumnutation rate changes of stems in three week-old Helianthus annuus. The growth and circumnutation were measured simultaneously. The course is a mean from 10 plants; the bars denote SE. The growth rate (dark line) is the smoothed (running average with a 1-hour window) first derivative that depicts only the long-time changes. The same holds true for the circumnutation movement rate (running average with a 1-hour window, gray line) too. The asterisks show the moments of the stem contractions presented in Figure 3B. The gray bars denote periods of darkness. The correlation coefficient between both rates is statistically significant and equals 0.5.
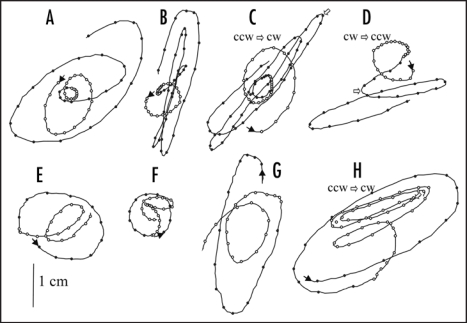
The typical top-view trajectories of circumnutation are shown in Figure 2. During the light stage the circumnutations were small, about 0.5–2 cm long; they had elliptical clockwise or anti-clockwise trajectories, and the rate of circumnutating was 0.05–0.15 mm min−1 (Figs. 1 and 2). In the dark stage the circumnutations were longer (3–6 cm), and usually of the rate in the range of 0.2–0.5 mm min−1 (Figs. 1 and 2).
Figure 2.
Examples of top view of stem circumnutation trajectory disturbances after darkening (A–D) and illumination (E–H) in Helianthus annuus. Open and dark circle lines denote the stem apex trajectories in light and dark, respectively. The points every 10 min. Light (70 µmol m2s−1) was applied from top at different phases of circumnutation. The reversion of the rotation direction (indicated by open arrows) took place and occurred as a pendular movement (H), or by a sharp curve at the “end” of the ellipse (C and D). ccw, counterclockwise; cw, clockwise. These examples represent 16 repetitions of switching the light off and on.
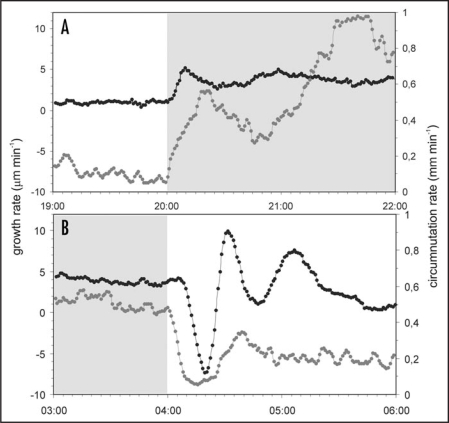
Besides long lasting fluctuations (in the range of many hours), there were light-induced short-term sunflower stem responses (in the range of a few minutes). After switching off the light (at 20:00), we observed a rapid increase in the growth rate, lasting about 10 minutes, which was accompanied by an increase in the circumnutation rate (Fig. 3A). The rate of circumnutation increased gradually but significantly, and their trajectories had various patterns presented in Figure 2A–D. In some cases the change of rotation direction was induced (Fig. 2C and D). After switching on the light (at 04:00), we observed a rapid decrease in the growth rate resulting in stem contraction (Fig. 3B, the plot beyond dashed line; in Fig. 1 the moment of stem contraction corresponds to the small decline marked by the asterisks and caused by a 1-hour window running average). The contraction was in average 64.7 ± 4.3 µm (n = 10) and persisted 12.8 ± 0.6 min (n = 10). The line presenting the kinetics of growth rate changes which were induced by switching on the light and lasted about 90 minutes had a characteristic wave-like shape (Fig. 3B). The stem contraction was accompanied by disturbances in the trajectory of circumnutation, which almost stopped for 10–15 minutes after switching on the light (Fig. 3B). Later the characteristic pattern of trajectory appeared (Fig. 2E–H). As a typical response, the stem apex made its way towards the centre of circumnutation. In some cases a subsequent change of rotation occurred (Fig. 2H).
Figure 3.
Circumnutation and growth rate of Helianthus annuus stem after darkening (A) and illumination (B). The typical example of the growth rate (dark line) and circumnutation rate (gray line) changes representing 10 repetitions. The gray bars denote periods of darkness.
Discussion
The above presented results show the relations between the growth rate and circumnutation rate of sunflower stem in a long- and short-time scale. As shown in Figure 1, under light:dark 16:8 h photoperiod both phenomena displayed a diurnal rhythm, but each of a different course. In the dark stage both had a high rate, whereas in the light stage the same growth rate was accompanied by a lower circumnutation rate. This suggests that light regulates growth and circumnutation in different ways. During 16 hours of illumination, light promoted growth but restricted circumnutation. This demonstrates that there is no simple relation between growth rate and circumnutation rate. Similar, non-proportional inhibition of growth and circumnutation due to the application of certain chemical compounds has been previously reported.16–18,24 In studies on aluminium toxicity in rice an inhibition of root-tip rotation was observed without a simultaneous inhibition of root elongation at a lower aluminium concentration. Thus, it was suggested that circumnutation inhibition is a new, early phase of response to aluminium stress.18 Another example is the change of circumnutation period and amplitude without altered growth rate after lithium treatment in Helianthus annuus seedlings and Phaseolus vulgaris shoots.16,17 Circumnutations were more severely inhibited than growth by red-light pulse in the coleoptiles of dark-grown rice seedlings.24 From this lack of proportion between changes of growth rate and circumnutation rate a general problem arises: is the circumnutation of a growing organ more sensitive to environmental factors than the growth itself ? The above mentioned examples suggest that the inhibition of circumnutation is a part of response to environmental factors, separate from growth inhibition. On the other hand, growth inhibition induced by a stronger stimulus, e.g., at higher aluminium concentrations, co-exists with the inhibition of circumnutation,18 and the growth inhibition resulting in sunflower stem contraction is accompanied by disturbances in the circumnutation trajectory.20 This, however, does not undermine the assumption that distortions and changes in circumnutations might play a role in the detection of environmental factors before growth inhibition itself takes place. We could therefore suggest a new physiological role for circumnutations, which would not merely be regarded as an inevitable consequence and way of growing. At this point another question related to the circumnutation mechanism emerges, namely: can reversible turgor changes occur during circumnutations? So far, the paper of Caré and al.,21 presenting reversible turgor changes of the cell length in circumnutating bean shoots, has been the only elegant confirmation of the issue. In our previous paper20 we showed that a stimulus accompanied by hydraulic changes may trigger circumnutation disturbances. The leaf was then heat-wounded, which induced a very rapid transient (half-life of about 30 s) stem elongation (5–15 µm) followed by stem contraction that lasted 40–60 minutes and was many micrometers long, sometimes exceeding 150 µm.19,25,26 The same stimulus also triggered disturbances in the period and trajectory of circumnutation.20 Our present results show a rapid decrease in the growth rate resulting in stem contraction accompanied by a transient pause and distortions in the circumnutation trajectory (Figs. 2 and 3); however, this time it was switching on the light that caused those phenomena. The light-induced contractions in a plant with a 260 mm-long stem were about 50 µm, which corresponds to 0.02% of the entire stem length. We have therefore demonstrated the ability of the sunflower stem tissues to contract in response to a light stimulus. This may be a next example of elastic properties of sunflower stem tissue. The transient character of the contraction may be due to elasticity of the cell wall, a property which allows the cell wall to contract around the (contracting) stem cell protoplast. The transient protoplast contractions, induced by blue light and accompanied by growth inhibition, were reported for Arabidopsis hypocotyls and maize coleoptiles.22,23,27 Light stimulus is able to induce cell contraction in such organs as hypocotyls and coleoptiles; therefore, we may assume that sunflower stem contraction presented in this paper may be accompanied by reversible turgor changes of cell volume. But do they also occur in the circumnutation mechanism in sunflower stem? This remains unclear and needs further investigation.
Besides the changes in circumnutation rate after switching the light on and off, we observed changes in the shape of circumnutation trajectories (Fig. 2). Baillaud28 presented top-view trajectories of nutating shoot apex of many plant species, Brown and al.29 demonstrated them in Helianthus hypocotyls apex, Hejnowicz and Sievers30—in tulip stem apex, and Schuster and Engelmann8 showed the trajectories of Arabidopsis hypocotyl. The interaction of circumnutation with phototropism and gravitropism was described by Orbovic and Poff.31 Changes in the circumnutation trajectory as a response to light stimulus have not been reported so far. The changes of circumnutation trajectories known from the literature are highly diverse and difficult to average.28 We, too, noticed a big variety and markedly distinctive character of individual trajectories, nevertheless, it is possible to distinguish the changes of circumnutation direction and also to see the trajectories directing towards the centre of circumnutations. The response in the form of various trajectories depends on the circumnutation phase, at which the stimulus was applied. It should be emphasized that the direction of circumnutation rotation and the trace drawn by the stem apex correspond to the direction and pattern of turgor changes which involve ion fluxes and circle around the “motor region”.16,32,33 Therefore, detailed analyses of the direction and shape of stem circumnutation and, especially, a study of the trajectory of direction changes could provide new information about the system of cell cooperation within the stem.
Conclusions
The time-lapse video system and positioning transducer were used to compare the kinetics of circumnutation and growth. Our experiments show two stages of helical movement: nocturnal-big and diurnal-small amplitude; the growth rate in both stages was similar. Thus, we concluded that the changes in circumnutation rate are not directly proportional to the changes in growth rate. Moreover, we observed that switching on the light induced a stem contraction, which could be the reason for a transient pause and disturbances of the circumnutation trajectory. This proves that a non-wounding stimulus, i.e., darkening and illumination triggers distinct and rapid turgor changes in the motor region of the sunflower stem. The above presented measurements of growth and circumnutation in sunflower stem contribute to the characteristics of the system of turgor changes34–37 which is likely to play an essential role in the integration and coordination of the cell functions in plant organism.
Materials and Methods
Plant materials and growth conditions.
The studies were carried out on three-week old Helianthus annuus L. plants grown in a vegetation room in pots filled with garden soil. They were watered with tap water and no other treatment was applied. A 16:8 h light:dark photoperiod was maintained in the vegetation room. The intensity of white light in PAR range (Power Star HQT-T400 W/D Osram) on the level of plant leaves was approximately 70 µmol m−2 s−1. The vegetation room was air-conditioned; the temperature was 24 ± 1°C and humidity 50–70%. Plants of 25 ± 0.6 cm (n = 33) height with two pairs of developed leaves were taken for the experiment.
Stem growth rate and circumnutation movements measurement.
The plants were transferred into the laboratory at 10:30. During the three-day long measurement the temperature in the lab room was 24 ± 1°C and the humidity 50–70%. Constant humidity was maintained in the pots, too. The plants were lighted by white light (Power Star HQT-T400 W/D Osram) of 70 µmol m−2 s−1 PAR intensity on the level of leaves. In the LD conditions the light was automatically switched off at 20:00 and on at 04:00 throughout the experiments. The stem growth rate changes were measured using angular position sensing transducers (Metripak Brush Instruments, Cleveland, OH, USA). The needle of the transducer was inserted across the stem in the fast growing region at 5 ± 1 cm below the stem apex (21 ± 1.5 cm above soil level). The true elongation of the stem, tracing along the curved stem itself towards the apex in a direction perpendicular to the soil was measured. The data acquisition was carried out for three days for every plant at 1 Hz sampling, each experiment started at 12:00. Insertion of the transducer needle 5 cm below the apex enabled the upper part of the stem to move and thus allowed simultaneous circumnutation recording by video camera. For circumnutation movement recording the time-lapse photography system was used. It consisted of a monochromatic CCD camera (0.01 lux sensitivity) with GV6V12 Ernitec F1, 4; 6–12 mm objectives working in a setup with a PC computer. Circumnutations were recorded by a camera placed above the plant. Recordings started at 12:00, and filming was conducted at one frame every 1 min. Trajectories described by the plant apex were recorded in a co-ordinate system in a horizontal, parallel to the soil plane. For thus observed circumnutations, circumnutation rate and circumnutation trajectory length were determined. For filming in darkness, a very dim green light of intensity <0.1 µmol m−2 s−1 PAR was used. The system was calibrated by a millimeter scale placed on the level of plant top and the resolution was 0.2 mm.
Data analysis.
To analyse the changes in growth rate we used a BiNoZ computer programme, which was a macro module in the Microsoft Excel programme, thanks to which we averaged the recording (assessed every second) at 1-min interval (60 experimental points). Next, the rate of the changes occurring within a minute was counted. In the presentation of long-term daily changes (Fig. 1) the course of growth rate was additionally smoothed by using the running average with 1-hour window. In the presentation of quick changes (Fig. 3) the course of growth rate was smoothed by the use of the running average with a 5-min window.
The time lapse images were digitized using Tracer (custom-made) and Microsoft Excel programmes. Experimental points (co-ordinates x, y of the stem apex trajectory) were determined at 5-min (Fig. 1) and 1-min (Fig. 3) intervals. Next, the rate of changes occurring within a minute was determined. In the presentation of long-term daily changes (Fig. 1) the course of circumnutation rate changes was smoothed using the running average with a 1-hour window. In the presentation of short-term changes (Fig. 3) the course of circumnutation rate was smoothed using the step average with a 5-min window. The distance covered by the stem apex during one full circumnutation was taken as the circumnutation trajectory length.9,20 The values of parameters are given as a mean ± SE. The present results were achieved from 10 plants in experiments where the measurement of growth and circumnutations was conducted simultaneously. Control measurements of circumnutations were also carried out on 10 plants in which the growth transducer was not inserted.
Acknowledgements
This work was supported by the grant from the Vice-Rector of Maria Curie-Sklodowska University.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5714
References
- 1.Johnsson A. Circumnutations: results from recent experiments on Earth and in space. Planta. 1997;203:147–158. doi: 10.1007/pl00008103. [DOI] [PubMed] [Google Scholar]
- 2.Engelmann W, Johnsson A. Rhythms in organ movement. In: Lumsden PJ, Millar AJ, editors. Biological rhythms and photoperiodism in plants. Oxford Washington: Bios Scientific Publishers; 1998. pp. 35–50. [Google Scholar]
- 3.Lecharny A, Wagner E. Stem extension rate in light-grown plants. Evidence for an endogenous circadian rhythm in Chenopodium rubrum. Physiol Plant. 1984;60:437–443. doi: 10.1104/pp.79.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prat R, Kellershohn N, Ricard J. Aperiodic (“Chaotic”) behaviour of plant cell wall extension-II Periodic and aperiodic oscillations of the elongation rate of a system of plant cells. Chaos, Solitons & Fractals. 1996;7:1119–1125. [Google Scholar]
- 5.Jouve L, Greppin H, Agosti RD. Arabidopsis thaliana floral stem elongation: Evidence for an endogenous circadian rhythm. Plant Physiol Biochem. 1998;36:469–472. [Google Scholar]
- 6.Dawson Day MJ, Millar AJ. Circadian dysfunction causes aberrant hypocotyls elongation patterns in Arabidopsis. Plant J. 1999;17:63–71. doi: 10.1046/j.1365-313x.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove DJ. Expansive growth of plant cell walls. Plant Physiol Biochem. 2000;38:109–124. doi: 10.1016/s0981-9428(00)00164-9. [DOI] [PubMed] [Google Scholar]
- 8.Schuster J, Engelmann W. Circumnutations of Arabidopsis thaliana seedlings. Biol Rhythm Res. 1997;28:422–440. [Google Scholar]
- 9.Buda A, Zawadzki T, Krupa M, Stolarz M, Okulski W. Daily and infradian rhythms of circumnutation intensity in Helianthus annuus. Physiol Plant. 2003;119:582–589. [Google Scholar]
- 10.Niinuma K, Someya N, Kimura M, Yamaguchi I, Hamamoto H. Circadian rhythm of circumnutation in inflorescence stems of Arabidopsis. Plant Cell Physiol. 2005;46:1423–1427. doi: 10.1093/pcp/pci127. [DOI] [PubMed] [Google Scholar]
- 11.Charzewska A, Zawadzki T. Circadian modulation of circumnutation length, period and shape in Helianthus annuus. J Plant Growth Regul. 2006;25:324–331. [Google Scholar]
- 12.Charzewska A. The rhythms of circumnutation in higher plants. In: Teixeira da Silva JA, editor. Floriculture ornamental and plant biotechnology: Advances and topical issues. Vol. 1. Department of Horticulare. Kagawa University; 2006. pp. 268–275. [Google Scholar]
- 13.Brown AH. Circumnutations: from Darwin to space flights. Plant Physiol. 1993;101:345–348. doi: 10.1104/pp.101.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg AR, Peacock K. Growth patterns in nutating and nonnutating sunflower (Helianthus annuus) hypocotyls. Am J Bot. 1992;79:77–85. [Google Scholar]
- 15.Johnsson A. Circumnutation. In: Haupt W, Feinleib E, editors. Encyclopedia of plant physiology, N S, vol 7, Physiology of Movements. Berlin: Springer; 1979. pp. 627–646. [Google Scholar]
- 16.Millet B, Badot PM. The revolving movement mechanism in Phaseolus: New approaches to old questions. In: Greppin H, Degli Agosti R, Bonzon M, editors. Vistas on Biorhythmicity. Geneva: University of Geneva; 1996. pp. 77–98. [Google Scholar]
- 17.Zachariassen E, Johnsson A. Effects of lithium ions on the circumnutations of Helianthus hypocotyls. Physiol Plant. 1988;72:147–152. [Google Scholar]
- 18.Hayashi Y, Nishiyama H, Tanoi K, Ohya T, Nihei N, Tanioka K, Nakanishi TM. An aluminium influence on root circumnutation in dark revealed by a new super-HARP (High-gain Avalanche Rushing Amorphous Photoconductor) camera. Plant Cell Physiol. 2004;45:351–356. doi: 10.1093/pcp/pch042. [DOI] [PubMed] [Google Scholar]
- 19.Stankovic B, Zawadzki T, Davies E. Characterization of the variation potential in sunflower. Plant Physiol. 1997;115:1083–1088. doi: 10.1104/pp.115.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stolarz M, Dziubinska H, Krupa M, Buda A, Trebacz K, Zawadzki T. Disturbances of stem circumnutations evoked by wound-induced variation potentials in Helianthus annuus L. Cell Mol Biol Lett. 2003;8:31–40. [PubMed] [Google Scholar]
- 21.Caré AF, Nefed'ev L, Bonnet B, Millet B, Badot PM. Cell elongation and revolving movement in Phaseolus vulgaris L. twining shoots. Plant Cell Physiol. 1998;39:914–921. doi: 10.1093/pcp/41.1.114. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Iino M. Blue-light-induced shrinking of protoplasts from maize coleoptiles and its relationship to coleoptiles growth. Plant Physiol. 1997;114:1009–1020. doi: 10.1104/pp.114.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Iino M. Interaction of cryptochrome1, phytochrome, and ion fluxes in blue-light-induced shrinking of Arabidopsis hypocotyl protoplasts. Plant Physiol. 1998;117:1265–1279. doi: 10.1104/pp.117.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshihara T, Iino M. Circumnutation of rice coleoptiles: its occurrence regulation by phytochrome, and relationship with gravitropism. Plant Cell Environ. 2005;28:134–146. doi: 10.1111/j.1365-3040.2004.01249.x. [DOI] [PubMed] [Google Scholar]
- 25.Davies E, Zawadzki T, Witters D. Electrical activity and signal transmission in plants: How do plants know? In: Penel C, Greppin H, editors. Plant signalling, plasma membrane, and change of state. Geneva: University of Geneva; 1991. pp. 119–137. [Google Scholar]
- 26.Stankovic B, Witters DL, Zawadzki T, Davies E. Action potentials and variation potentials in sunflower: An analyssis of their relationships and distinguishing characteristics. Physiol Plant. 1998;103:51–58. [Google Scholar]
- 27.Parks MB, Cho MH, Spalding EP. Two genetically separable phases of growth inhibition induced by blue light in Arabidopsis seedlings. Plant Physiol. 1998;118:609–615. doi: 10.1104/pp.118.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baillaud L. Mouvements autonomes des tiges, vrilles et autres organes à l'exception des organes volubiles et des feuilles. In: Ruhland W, editor. Handbuch der Pflanzenphysiologie 17/2. Berlin: Springer-Verlag; 1962. pp. 562–634. (Ger). [Google Scholar]
- 29.Brown A, Chapman DK, Lewis RF, Venditti Circumnutations of sunflower hypocotyls in satellite orbit. Plant Physiol. 1990;94:233–238. doi: 10.1104/pp.94.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hejnowicz Z, Sievers A. Proton efflux from the outer layer of the peduncle of tulip in gravitropism and circumnutation. Bot Acta. 1995;108:7–13. [Google Scholar]
- 31.Orbovic V, Poff L. Interaction of light and gravitropism with nutation of hypocotyls of Arabidopsis thaliana seedlings. Plant Growth Regul. 1997;23:141–146. doi: 10.1023/a:1005853128971. [DOI] [PubMed] [Google Scholar]
- 32.Shabala SN, Newman IA. Proton and calcium flux oscillations in the elongation region correlate with root nutation. Physiol Plant. 1997;100:917–926. [PubMed] [Google Scholar]
- 33.Shabala SN, Newman IA. Root nutation modelled by two ion flux-linked growth waves around the root. Physiol Plant. 1997;101:770–776. [Google Scholar]
- 34.Wagner E, Normann J, Albrechtová J, Bonzon M, Greppin H. Photoperiodic control of flowering: electrochemical-hydraulic communication between plant organs—“Florigen” a frequency-coded electric signal? In: Greppin H, Penel C, Simon P, editors. Travelling shot on plant development. Geneva: University of Geneva; 1997. pp. 19–34. [Google Scholar]
- 35.Wagner E, Lehner L, Normann J, Veit J, Albrechtová J. Hydro-electrochemical integration of the higher plant—basis for electrogenic flower induction. In: Baluška F, Mancuso S, Volkmann D, editors. Communication in plants. Berlin Heidelberg: Springer-Verlag; 2006. pp. 369–389. [Google Scholar]
- 36.Peters WS, Hagemann W, Tomos AD. What makes plant different? Principles of extracellular matrix function in “soft” plant tissues. Comp Bioch Physiol A. 2000;125:151–167. doi: 10.1016/s1095-6433(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 37.Nardini A, Salleo S, Andri S. Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot) Plant Cell Environ. 2005;28:750–759. [Google Scholar]