Abstract
Aggregation of proteins damaged by stress is often a causal factor of cell death. To prevent aggregation, eukaryotic cells rapidly degrade damaged proteins by engaging two types of proteasomes. The first type is the 26S proteasome (26SP) which is composed of a cylindrical proteolytic core—the 20S proteasome (20SP)—and one or two regulatory particles (RPs) that interact with ubiquitinated proteins. The second type is the free 20SP which mediates ubiquitin-independent proteolysis. We have recently shown that loss of RP function in Arabidopsis leads to an expected decrease in 26SP-dependent protein degradation and hypersensitivity to stresses that induce protein misfolding. Surprisingly, RP mutants have increased 20SP activity and tolerance to oxidative stress. This finding suggests that misfolded proteins carry one type of degradation signal that steers them to ubiquitination enzymes and the 26SP, while oxidatively damaged proteins carry another that guides them directly to the 20SP for degradation. Here we suggest that protein oxidation induces the formation of unstructured regions that serve as targeting signals for 20SP-dependent proteolysis.
Key words: 20S proteasome, misfolded proteins, oxidized proteins, ubiquitin-independent proteolysis, unstructured regions
Proteasomes are an essential component of the quality-control system that limits the accumulation of non-functional proteins in the cell.1–4 A protein can be rendered non-functional by mutations, translational and folding errors, and adverse conditions such as heat shock and oxidative stress. These proteins decrease the efficiency of metabolic pathways not only because of their loss of function, but also because of the deleterious gain-of-function effects generally known as proteotoxicity.1–4 Until recently, it was widely accepted that the detection and degradation of all non-functional proteins is initiated by their loss of native tertiary structure followed by misfolding. Misfolding is believed to expose hydrophobic regions that form interaction domains for chaperones, which are in turn bound to ubiquitin ligases that label the target for 26SP-dependent proteolysis.5–9 Thus, the degradation of proteins that have lost their native conformation was considered to be an ubiquitin (Ub)- and 26SP-dependent process (Fig. 1).
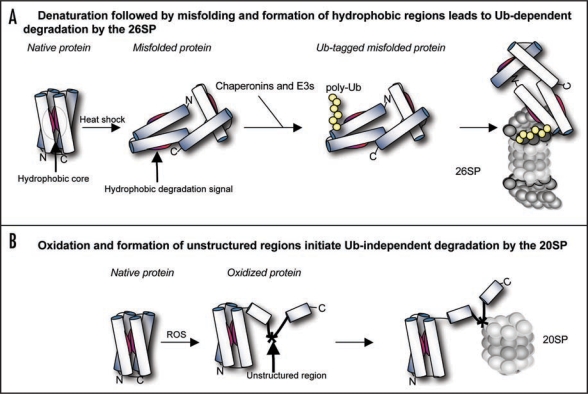
Figure 1.
Model for the degradation of damaged proteins. (A) Stresses such as heat shock or the incorporation of amino acid analogues induce protein misfolding. If for example, a globular protein is misfolded, its hydrophobic core will be exposed to the cytoplasm. These hydrophobic regions can bind chaperones that either repair the misfolded protein or shuttle it to the ubiquitination enzymes and the 26SP. (B) Protein oxidation leads to a partial loss of secondary structure without disrupting the overall folding pattern of the protein, resulting in flexible, unstructured regions. These regions serve as degradation signals for the Ub-independent 20SP pathway.
However, a number of studies have shown that the degradation of proteins damaged by oxidative stress follows another route: oxidized proteins are degraded by the 20SP in a Ub-independent manner.5–7,10 We have recently shown that this proteolysis pathway is important for oxidative stress tolerance in plants. Loss of function of the Arabidopsis RP subunits RPT2a, RPN10 and RPN12a reduces 26SP function and leads to an expected decrease in Ub-dependent proteolysis.11–14 Unexpectedly, all three RP mutants have increased 20SP activity, which is probably caused by the stabilization of an activator of proteasome biogenesis that is normally degraded by the 26SP.11 This shift in proteasome activity leads to increased oxidized protein turnover and oxidative stress tolerance, but also to decreased tolerance to stresses that are known to cause protein misfolding.11 Thus, the 26SP in Arabidopsis is needed for the removal of misfolded proteins, and the 20SP is essential for the degradation of oxidized proteins.
This differential degradation of damaged proteins implies that plant cells have distinct recognition mechanisms for misfolded and oxidized proteins, and that oxidation leads to the formation of a specific degradation signal that channels the oxidized proteins directly to the 20SP. Nevertheless, it has been suggested that the proteolysis of oxidized proteins also depends on misfolding and exposure of hydrophobic regions that serve as recognition sites for either the 20SP itself or for specific chaperonins that bind the 20SP.5–7,10,15 If the recognition of proteasomal targets is specific, then—according to the current theory—heat shock and oxidative stress would expose specific types of hydrophobic degradation signals in any cellular protein. These qualitatively different hydrophobic regions would lead either to ubiquitination and 26SP-dependent degradation or to a direct interaction with the 20SP. While we cannot exclude this, it is hard to envision how a random process such as misfolding would produce discernable degradation signals dependent on whether the denaturation was caused by heat shock or by oxidation. An alternative explanation is that the recognition of oxidized proteins does not depend on misfolding.
How would oxidized proteins then be targeted to the 20SP? Today we know of some functional proteins that are degraded by the 20SP in a Ub-independent manner, and all these characterized 20SP targets have regions that lack secondary structure.10,16 The native unstructured regions or intrinsically disordered regions give conformational plasticity to a protein and allow it to form a complex with different partners.17,18 The unstructured regions are also thought to serve as initiation sites for proteolysis.19 These findings are a starting point for the “degradation by default” theory which states that many proteins in their native conformation contain unstructured regions that make them inherently unstable and target them to the Ub-independent 20SP pathway.10 Such proteins tend to be stabilized by forming complexes in which the unstructured regions are masked by other polypeptides. Since oxidized proteins are processed by the 20SP, their common degradation signal could also be an intrinsically disordered region (Fig. 1). Thus, protein oxidation—at least mild protein oxidation6—would lead to the formation of flexible peptide stretches (i.e., unstructured regions) rather than to protein misfolding (i.e., unfolding and non-native refolding that exposes otherwise sequestered hydrophobic residues). This hypothesis is supported by a study of the 20SP-dependent degradation of oxidized calmodulin (CaM).20 Oxidation of CaM leads to a significant increase in its 20SP-dependent and Ub-independent degradation. In vitro studies revealed a positive correlation between decreased secondary structure (i.e., increased flexibility) and proteolysis rate, and no correlation between changes in surface hydrophobicity and CaM stability.20
There is another paradox concerning 20SP-dependent proteolysis of oxidized proteins. The 20SP is a barrel-shaped particle composed of two α and two β rings in an α7β7b7β7 configuration.21 Proteolytic activity is confined to the β rings and is broad range, so that it degrades any target into oligopeptides of 3–25 amino acids in length. To be degraded, targets must not only be recognized by the 20SP, but must also enter into the proteolytic chamber through a constriction in the α rings known as the α-annulus. In 26SP-dependent proteolysis, this entry point is opened by the action of a ring of AAA ATPases from the RP.21 However, this entrance gate of the free 20SP is closed and restricts random proteolysis. How then do oxidized proteins enter the proteolytic chamber? It has been shown that some natively unstructured proteins can open the gates possibly by acting as chaotropes and by causing subunit residues to become disordered.22 This then could also be the entry mechanism for oxidized proteins.
In conclusion, analyses of Arabidopsis proteasome mutants with decreased Ub-dependent proteolysis reveals that the 20SP-dependent “degradation by the default” pathway is operational in plants and is important for oxidative stress tolerance. However, it remains to be shown whether indeed the unstructured regions, either innate or formed by the action of free radicals, guide proteins to the 20SP and specifically cause the opening of the α-annulus. The identities of native 20SP targets in plants also await further studies.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5376
References
- 1.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 2.Esser C, Alberti S, Hohfeld J. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim Biophys Acta. 2004;1695:171–188. doi: 10.1016/j.bbamcr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 4.Ron D. Stressed cells cope with protein overload. Science. 2006;313:52–53. doi: 10.1126/science.1130469. [DOI] [PubMed] [Google Scholar]
- 5.Voss P, Grune T. The nuclear proteasome and the degradation of oxidatively damaged proteins. Amino Acids. 2007;32:527–534. doi: 10.1007/s00726-006-0428-5. [DOI] [PubMed] [Google Scholar]
- 6.Jung T, Bader N, Grune T. Oxidized proteins: Intracellular distribution and recognition by the proteasome. Arch Biochem Biophysics. 2007;462:231–237. doi: 10.1016/j.abb.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Grune T, Jung T, Merker K, Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid and ‘aggresomes’ during oxidative stress, aging and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch C, Gauss R, Sommer T. Coping with stress: cellular relaxation techniques. Trends Cell Biol. 2006;16:657–663. doi: 10.1016/j.tcb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nature Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 10.Asher G, Reuven N, Shaul Y. 20S proteasomes and protein degradation “by default”. Bioessays. 2006;28:844–849. doi: 10.1002/bies.20447. [DOI] [PubMed] [Google Scholar]
- 11.Kurepa J, Toh-e A, Smalle J. 26S proteasome regulatory particle mutants have increased oxidative stress tolerance. Plant J. 2008;53:102–114. doi: 10.1111/j.1365-313X.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 12.Smalle J, Kurepa J, Yang P, Babiychuk E, Kushnir S, Durski A, Vierstra RD. Cytokinin growth responses in Arabidopsis involve the 26S proteasome subunit RPN12. Plant Cell. 2002;14:17–32. doi: 10.1105/tpc.010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smalle J, Kurepa J, Yang P, Emborg TJ, Babiychuk E, Kushnir S, Vierstra RD. The pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in abscisic acid signaling. Plant Cell. 2003;15:965–980. doi: 10.1105/tpc.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda M, Matsui K, Ishiguro S, Sano R, Wada T, Paponov I, Palme K, Okada K. The HALTED ROOT gene encoding the 26S proteasome subunit RPT2a is essential for the maintenance of Arabidopsis meristems. Development. 2004;131:2101–2111. doi: 10.1242/dev.01096. [DOI] [PubMed] [Google Scholar]
- 15.Whittier JE, Xiong Y, Rechsteiner MC, Squier TC. Hsp90 enhances degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem. 2004;279:46135–46142. doi: 10.1074/jbc.M406048200. [DOI] [PubMed] [Google Scholar]
- 16.Verma R, Deshaies RJ. A proteasome howdunit: the case of the missing signal. Cell. 2000;101:341–344. doi: 10.1016/s0092-8674(00)80843-0. [DOI] [PubMed] [Google Scholar]
- 17.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 18.Schlessinger A, Punta M, Rost B. Natively unstructured regions in proteins identified from contact predictions. Bioinformatics. 2007;23:2376–2384. doi: 10.1093/bioinformatics/btm349. [DOI] [PubMed] [Google Scholar]
- 19.Prakash S, Tian L, Ratliff KS, Lehotzky RE, Matouschek A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat Struct Mol Biol. 2004;11:830–837. doi: 10.1038/nsmb814. [DOI] [PubMed] [Google Scholar]
- 20.Ferrington DA, Sun H, Murray KK, Costa J, Williams TD, Bigelow DJ, Squier TC. Selective degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem. 2001;276:937–943. doi: 10.1074/jbc.M005356200. [DOI] [PubMed] [Google Scholar]
- 21.Kurepa J, Smalle J. Structure, function and regulation of plant proteasomes. Biochimie. 2007 doi: 10.1016/j.biochi.2007.07.019. in press. [DOI] [PubMed] [Google Scholar]
- 22.Förster A, Hill CP. Proteasome degradation: enter the substrate. Trend Cell Biol. 2003;13:550–553. doi: 10.1016/j.tcb.2003.09.001. [DOI] [PubMed] [Google Scholar]