Abstract
Development of sessile organisms requires adaptation to an ever-changing environment. In order to respond quickly to these challenges, complex signaling mechanisms have evolved to facilitate cellular modifications. The importance of phospholipid-based signaling pathways in plants, as well as animals, has recently been gaining attention. Both the PLD and PLC pathways produce the signaling molecule PA, which modulates MTs, F-actin and endomembrane trafficking. We have examined the roles of the PLD signaling pathway during development of the marine brown alga Silvetia compressa. Zygotes were treated with 1- and 2-butanol, both of which activate the PLD enzyme. However, only 1-butanol competes with water as a transphosphatidylation substrate, at the expense of PA production. Interestingly, we found that 1- and 2-butanol both disrupted MT organization and thereby cell division, with 1-butanol being more potent. These findings question whether the effects of butyl alcohol treatment are due to lowered PA levels or activation of the PLD enzyme. Additionally, preliminary results show that inhibition of DAGK results in loss of centrosomal MTs and formation of cortical MT cages that are strikingly similar to those formed following 1-butanol treatment. These data suggest that perturbation of the PLD or PLC pathway leads to cortical stabilization and/or nucleation of MT arrays.
Key words: actin, brown algae, cytoskeleton, development, endomembrane, microtubule, phosphatidic acid, phospholipase C, phospholipase D, stramenopile
Phosphatidic Acid Production
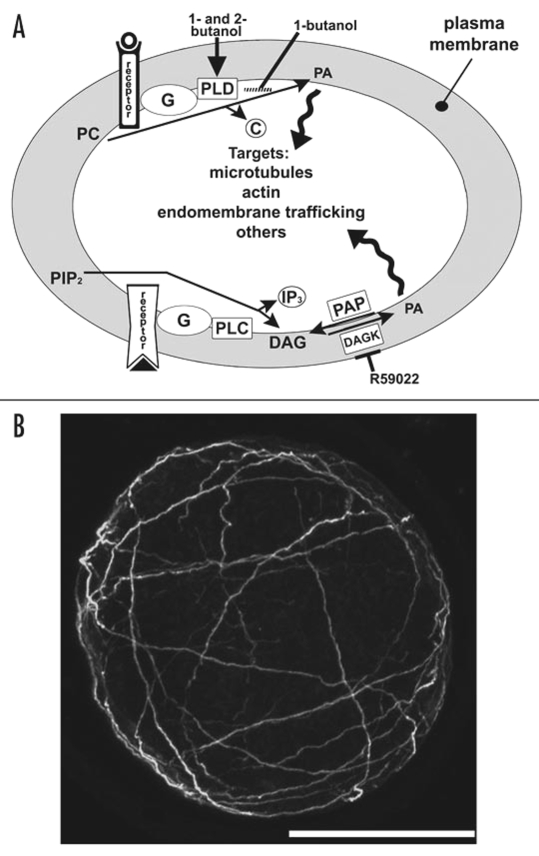
Phosphatidic acid (PA) is a membrane-localized signaling molecule that can be produced through two distinct pathways (Fig. 1A). The phospholipase D (PLD) pathway produces PA by hydrolyzing structural phospholipids, primarily phosphatidyl choline (PC) and phosphtidylethanolamine (PE).1 The phospholipase C (PLC) pathway cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and DAG, which is phosphorylated by diacylgylcerol kinase (DAGK), yielding PA.2 Dephosphorylation of diacylglycerol pyrophosphate (DGPP, not shown) also produces PA.2
Figure 1.
(A) The Phospholipase D (upper) and C (lower) pathways. Extracellular signaling activates G-protein coupled receptors; heterotrimeric G-proteins then activate PLD and PLC. PLD and PLC synthesize PA as described in the text. Both 1-butanol and 2-butanol disrupt the PLD pathway by mimicking receptor binding, while conversion of DAG to PA can be blocked by R59022, which inhibits DAGK activity. (B) Projection of confocal sections from the nucleus to the cortex of a zygote treated with 20 µM R59022 at 1 h AF, fixed and immunolabeled for MTs at 24 h AF. Scale bar equals 50 µm.
Phospholipase D Signaling
PLD signaling in animals regulates vesicle trafficking and organization of actin arrays.1 In Arabidopsis, which has 12 PLD genes, it has proven difficult to isolate mutants with aberrant phenotypes.3 Instead, chemical disrupters such as 1-butanol have provided valuable information about PLD signaling functions.4 A myriad of cellular and developmental processes such as germination,5 cell elongation,5 senescence6 and many stress responses are regulated by PLD signaling in plants.7–9 At the subcellular level, treatment of plant cells with 1-butanol leads to defects in actin arrays and endomembrane organization as in animals,10–12 and also disorganizes cortical MT arrays.13,14
We recently examined the roles of PLD signaling during early development of the brown alga S. compressa.15 A fertilized egg normally orients its growth axis in accordance with directional light (photopolarization) and germinates and grows from the rhizoid pole of that axis.16 The first division is asymmetric and is oriented transverse to the growth axis.17 We found that butanol treatments did not block photopolarization or germination, but cell division was inhibited.15 This suggested that MTs, rather than actin or endomembranes, were the primary targets of drug treatment. This was a somewhat surprising finding since actin is often disrupted by 1-butanol application in plants and animals.1,10,11 MT arrays in treated zygotes were examined by confocal microscopy. In untreated zygotes, the MT array is nucleated from perinuclear centrosomes and extends to the cell cortex. Following treatment, MTs initially appeared fragmented and, within two hours, became heavily bundled and resided exclusively in the cortex.15 Treated algal zygotes ultimately arrested in mitosis, unable to form a bipolar metaphase spindle. MT arrays and development recovered quickly following 1-butanol removal, providing an easy method for synchronizing populations. Of particular interest, we found that application of higher levels of 2-butanol mimicked the effects of 1-butanol. This observation has not been described in other systems. While 1-butanol is known to activate PLD and compete with water as the transphosphatidylation substrate, 2-butanol only activates PLD.4 These findings therefore question whether 1-butanol acts exclusively by lowering PA levels.
Two models have been proposed to explain the observed effects of 1-butanol treatment. The first model suggests that 1-butanol treatment leads to dramatically lowered PA levels, thereby disrupting signaling and resulting in cellular and developmental defects.5 This model is supported by studies showing that exogenous addition of PA rescues the effects of 1-butanol treatment.18,19 However, to date there is no direct evidence showing a decrease in PA levels following 1-butanol application. The second model is based on work in higher plants showing PLD decoration of cortical interphase MTs and also showing PLD in close association with the plasma membrane.20 In this model, activation of PLD by 1-butanol facilitates release of MTs from membrane-bound PLD, thereby disrupting MT organization and subsequently causing developmental defects.13 To discriminate these models, we are currently performing radio-labeling experiments to determine whether treatments with 1- or 2-butanol reduces the level of PA derived from the PLD pathway, and immunolabeling experiments to examine the spatial relationship between MTs and PLD.
Phospholipase C Signaling
The PLC pathway produces IP3, which regulates Ca2+ release from intracellular stores,2 and DAG, which can be phosphorylated by DAGK to yield PA.2 Mammalian DAGK has been shown though gene knockouts and chemical inhibition to function in regulation of Rac1 activity during membrane ruffling, neural and immune responses, cell proliferation and carcinogenesis.21 In higher plants, DAGK function is not well understood. However, treatment with R59022, a chemical inhibitor of DAGK, inhibits root elongation and lateral root formation in Arabidopsis.22
We have very recently begun to examine the effects of R59022 on zygotic development in S. compressa and preliminary findings indicate that germination, division and MT arrays are severely disrupted by drug treatment. Following R59022 treatment, MTs form a cortical cage and no MTs are found near centrosomes (Fig. 1B). Interestingly, 1-butanol also eliminates centrosomal MTs and induces formation of a bundled cortical array,15 but the significance of these localizations is presently unclear. PA derived from the PLC-pathway likely signals to more than MTs, since R59022 blocks germination, which does not require MTs. We are now examining the organization of filamentous actin arrays and the endomembrane system in R59022-treated zygotes, as well as determining PA levels derived from the PLC pathway. These studies will be reported in detail elsewhere.
Perspectives
We find that, as in plants and animals, the PLD and PLC pathways play fundamental roles in brown algal development. However, the formation of cortical MTs following perturbation of the pathways appears to be a unique observation. Why would disruption of PLD signaling or inhibition of DAGK lead to loss of centrosomal MT arrays and formation of a bundled cortical array? While centrosomally-nucleated MT arrays in brown algae have been shown to extend to the cortex and extend along it, the presence of cortical MTs arrays has only recently been reported. In F. serratus, a closely related brown alga, injection of fluorescent tubulin visualized centrosomal MT arrays as well as arrays residing solely in the cortex.23 Together, these data suggest that (1) MT arrays can be stabilized by interactions with the plasma membrane, and (2) the plasma membrane may be capable of MT nucleation. Interestingly, cortical MT nucleation and MT stabilization by interaction with the plasma membrane are both characteristic of higher plant interphase MTs.24 Further examination and understanding of phospholipid signaling in brown algae will provide valuable insights into how PLD and PLC pathways regulate development, and will illuminate how these pathways have evolved in different lineages.
Abbreviations
- DAG
diacylglycerol
- DAGK
diacylgylcerol kinase
- DGPP
diacylglycerol pyrophosphate
- IP3
inositol 1,4,5-triphosphate
- PA
phosphatidic acid
- PC
phosphatidyl choline
- PE
phosphatidylethanolamine
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PLC
phospholipase C
- PLD
phospholipase D
- MT
microtubule
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5419
References
- 1.Oude Weernink P, López de Jesús M, Schmidt M. Phospholipase D signaling: orchestration by PIP2 and small GTPases. Naunyn-Schmiedeberg's Arch Pharmacol. 2007;374:399–411. doi: 10.1007/s00210-007-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X. Lipid signaling. Cur Opin Plant Biol. 2004;7:329–336. doi: 10.1016/j.pbi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Munnik T, Musgrave A. Phospholipid signaling in plants: holding on to phospholipase D. Sci STKE. 2001 doi: 10.1126/stke.2001.111.pe42. PE42. [DOI] [PubMed] [Google Scholar]
- 4.Munnik T, Arisz SA, de Vrije T, Musgrave A. G protein activation stimulates phospholipase D signaling in plants. Plant Cell. 1995;7:2197–2210. doi: 10.1105/tpc.7.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardiner J, Collings DA, Harper JDI, Marc J. The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organization in Arabidopsis. Plant Cell Physiol. 2003;44:687–696. doi: 10.1093/pcp/pcg095. [DOI] [PubMed] [Google Scholar]
- 6.Meijer H, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biol. 2003;54:265–306. doi: 10.1146/annurev.arplant.54.031902.134748. [DOI] [PubMed] [Google Scholar]
- 7.de Jong CF, Laxalt A, Bargmann BOR, de Wit PJGM, Joostein MHAJ, Munnik T. Phosphatidic acid accumulation is an early response in the Cf-4/Avr4 interaction. The Plant J. 2004;39:1–12. doi: 10.1111/j.1365-313X.2004.02110.x. [DOI] [PubMed] [Google Scholar]
- 8.Vergnolle C, Vaultier MN, Taconnat L, Renou JP, Kader JC, Zachowski A, Ruelland E. The cold-induced early activation of phospholipase C and D pathways determines the response of two distinct clusters of genes in Arabidopsis cell suspensions. Plant Physiol. 2005;139:1217–1233. doi: 10.1104/pp.105.068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Hartog M, Verhoef N, Munnik T. Nod factor and elicitors activate different phospholipid signaling pathways in suspension-cultured alfalfa cells. Plant Physiol. 2003;132:311–317. doi: 10.1104/pp.102.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motes CM, Pechter P, Yoo CM, Wang YS, Chapman KD, Blancaflor EB. Differential effects of two phospholipase D inhibitors, 1-butanol and N-acylethanolamine, on in vivo cytoskeletal organization and Arabidopsis seedling growth. Protoplasma. 2005;226:109–123. doi: 10.1007/s00709-005-0124-4. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Gao L, Blanchoin L, Staiger CJ. Heterodimeric capping protein from Arabidopsis is regulated by phosphatidic acid. Mol Biol Cell. 2006;17:1946–1958. doi: 10.1091/mbc.E05-09-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montiero D, Liu Q, Lisboa S, Scherer G, Quader H, Malho R. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c, membrane secretion and the actin cytoskeleton. J Exp Bot. 2005;56:1665–1674. doi: 10.1093/jxb/eri163. [DOI] [PubMed] [Google Scholar]
- 13.Dhonukshe P, Laxalt AM, Goedhart J, Gadella TWJ, Munnik T. Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell. 2003;15:2666–2679. doi: 10.1105/tpc.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirase A, Hamada T, Itoh TJ, Shimmen T, Sonobe S. n-Butanol induces depolymerization of microtubules in vivo and in vitro. Plant Cell Physiol. 2006;47:1004–1009. doi: 10.1093/pcp/pcj055. [DOI] [PubMed] [Google Scholar]
- 15.Peters NT, Logan KO, Miller AC, Kropf DL. Phospholipase D signaling regulates microtubule organization in the fucoid alga Silvetia compressa. Plant Cell Physiol. 2007;48:1764–1774. doi: 10.1093/pcp/pcm149. [DOI] [PubMed] [Google Scholar]
- 16.Kropf DL, Bisgrove SR, Hable WE. Establishing a growth axis in fucoid algae. Trends Plant Sci. 1999;4:490–494. doi: 10.1016/s1360-1385(99)01509-5. [DOI] [PubMed] [Google Scholar]
- 17.Bisgrove SR, Kropf DL. Asymmetric cell divisions: Zygotes of fucoid algae as a model system. In: Verma DPS, Hong Z, editors. Cell Division Control in Plants. Heidelberg: Springer-Verlag; 2007. Volume in press, Plant Cell Monographs. [Google Scholar]
- 18.Komis G, Quader H, Galatis B, Apostolakos P. Macrotubule-dependent protoplast volume regulation in plasmolysed root-tip cells of Triticum turgidum: involvement of phospholipase D. New Phytol. 2006;171:737–750. doi: 10.1111/j.1469-8137.2006.01784.x. [DOI] [PubMed] [Google Scholar]
- 19.Potocký M, Eliáš M, Profotová B, Novotná Z, Valentová O, Žárský V. Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta. 2003;217:122–130. doi: 10.1007/s00425-002-0965-4. [DOI] [PubMed] [Google Scholar]
- 20.Gardiner J, Harper JD, Weerakoon N, Collings DA, Ritchie S, Gilroy S, Cyr RJ, Marc J. A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell. 2001;13:2143–2158. doi: 10.1105/TPC.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakane F, Imai SI, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases: Why so many of them? Biochim Biophys Acta-Mol Cell Biol Lipids. 2007;1771:793–806. doi: 10.1016/j.bbalip.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Gomez Merino FC, Arana Ceballos FA, Trejo Tellez LI, Skirycz A, Brearley CA, Dormann P, Mueller Roeber B. Arabidopsis AtDGK7, the smallest member of plant diacylglycerol kinases (DGKs), displays unique biochemical features and saturates at low substrate concentration. J Biol Chem. 2005;280:34888–34899. doi: 10.1074/jbc.M506859200. [DOI] [PubMed] [Google Scholar]
- 23.Corellou F, Coelho SMB, Bouget FY, Brownlee C. Spatial re-organization of cortical microtubules in vivo during polarisation and asymmetric division of Fucus zygotes. J Cell Sci. 2005;118:2723–2734. doi: 10.1242/jcs.02353. [DOI] [PubMed] [Google Scholar]
- 24.Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, Horio T, Hasebe M. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat Cell Biol. 2005;7:961–968. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]