Abstract
Salinity causes billion dollar losses in annual crop production. So far, the main avenue in breeding crops for salt tolerance has been to reduce Na+ uptake and transport from roots to shoots. Recently we have demonstrated that retention of cytosolic K+ could be considered as another key factor in conferring salt tolerance in plants. A subsequent study has shown that Na+-induced K+ efflux in barley root epidermis occurs primarily via outward rectifying K+ channels (KORC). Surprisingly, expression of KORC was similar in salt- tolerant and sensitive genotypes. However, the former were able to better oppose Na+-induced depolarization via enhanced activity of plasma membrane H+-ATPase (thus minimizing K+ leak from the cytosol). In addition to highly K+-selective KORC channels, activities of several types of non-selective cation channels were detected at depolarizing potentials. Here we show that the expression of one of them, NORC, was significantly lower in salt-tolerant genotypes. As NORC is capable of mediating K+ efflux coupled to Na+ influx, we suggest that the restriction of its activity could be beneficial for plants under salt stress.
Key words: salinity tolerance, barley, ion flux, K+ homeostasis, KOR, non-selective channels, patch-clamp
Reduced Na+-Induced K+ Efflux from Barley Roots is due to a Better Control of Membrane Potential and Higher H+-Pump Activity
An early plant response to NaCl is the influx of Na+ into root epidermal cells through non-selective channels at the plasma membrane, followed by a depolarization and activation of the K+ efflux.1 Although the mechanisms of Na+ toxicity are largely due to replacement of K+ by Na+ in metabolically active compartments and there is an obvious coupling between Na+ uptake and K+ efflux, traditionally the mechanisms of Na+ exclusion were considered much more important for salt tolerance than the mechanisms by which stressed plants retain intracellular K+.2–5 Recent studies on barley roots, however, point out that the magnitude of the K+ efflux induced by salt inversely correlate with the productivity of 62 out of 69 cultivars contrasting in their sensitivity to salt stress.6,7 The remaining seven cultivars achieved a maintenance of cytosolic K+/Na+ homeostasis via better Na+ exclusion. These results imply that, although the role of Na+ exclusion is indeed important for crop salt tolerance, its significance seems to be overestimated. On the contrary, the plant's ability to retain K+ in the cytosol has been substantially overlooked.
Using electrophysiological techniques (MIFE, impalement microelectrodes, patch-clamp) together with monitoring the unilateral Na+ uptake and H+-ATPase assays, we attempted to establish the molecular basis of Na+-induced K+ efflux from barley roots.8 We have shown that salt-tolerant genotypes of barley possess intrinsically higher plasma membrane H+ pump activity, despite having the same level of H+-ATPase expression. In accordance with this, salt-tolerant barley plants displayed a better control of membrane potential in the presence of high NaCl in the external medium. 80 mM Na+ treatment induced membrane potential depolarization to −50 – −60 mV in salt- sensitive barley plants, while in tolerant cultivars they were in the −75 – −85 mV range. The resultant 25 mV difference implicates a 3-fold lower K+ efflux via depolarization-activated KORC, matching the difference in observed net fluxes of K+ induced by NaCl in salt-sensitive vs salt-tolerant cultivars. At the same time, neither the average current density of KORC per epidermal protoplast, nor its voltage dependence, were significantly different in cultivars contrasting in their salt sensitivity.8
There are Multiple Routes of Na+-K+ Exchange at Plasma Membrane
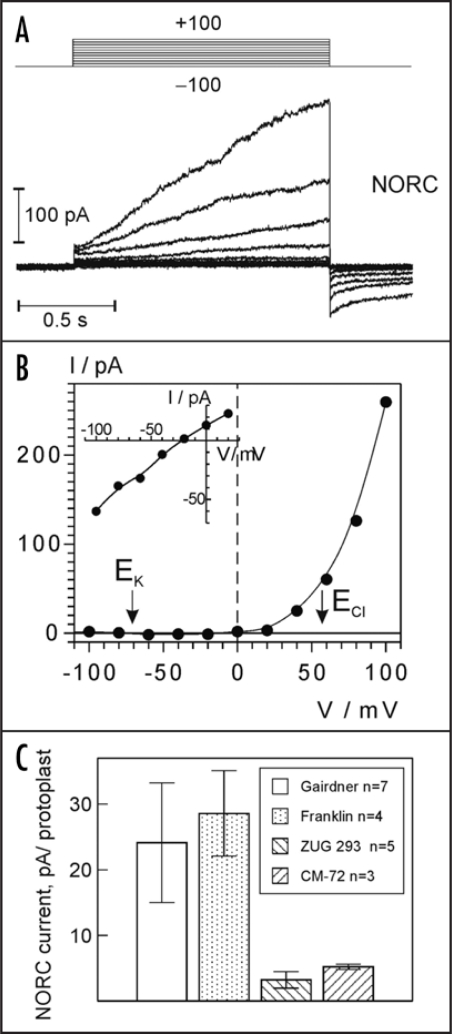
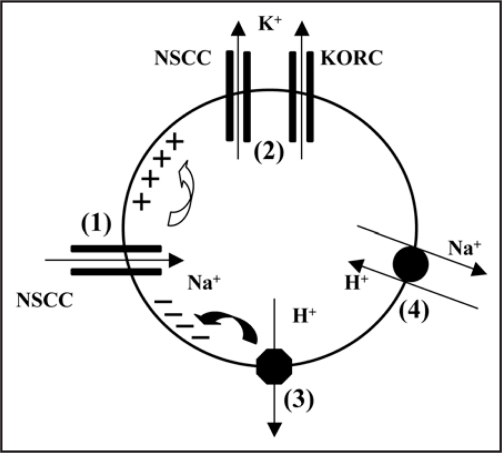
Patch-clamp studies of isolated barley epidermal protoplasts revealed at least four cation conductances present at depolarizing potentials. Based on the voltage- and time-dependent kinetics, one of them (depicted in Fig. 1), closely resembled the so-called nonselective outward-rectifying NORC channel, previously described in barley root xylem parenchyma cells.9,10 Compared to KORC, NORC displays a weaker voltage sensitivity, and, importantly, poorer selectivity for cations over anions, manifested by a reversal potential well above EK (see inset in Fig. 1B). Its xylem ortologue conducted Ca2+ and small monovalent cations (Na+, K+) with little preference, and displayed also a limited Cl− permeability.10 As shown in Fig. 1C, the expression of NORC-like currents in root epidermal protoplasts is reciprocally related to salt tolerance, i.e., tolerant cultivars expressed on average a 5-fold smaller NORC currents compared to sensitive ones. As NORC is permeable to both Na+ and K+, it can mediate both Na+ influx and K+ efflux (Fig. 2). Therefore, a suppression of this activity under salt stress may be beneficial for maintaining a high cytosolic K+/ Na+ ratio. A simplified model of plasma membrane Na+ and K+ transport under salt stress conditions is presented in Figure 2. Na+ enters the cytosol via non-selective cation channels (the relative importance of discovered NSCCs has to be determined) and depolarizes the membrane, provoking K+ loss via KORC and NSCCs (e.g., NORC). The activity of plasma membrane H+-ATPase opposes NaCl-induced membrane depolarization and its related K+ efflux. Besides, it fuels the Na+/H+ antiport, further improving the cytosolic Na+/K+ ratio.
Figure 1.
Non-selective outward current in barley root epidermal protoplasts. (A) Typical current traces of NORC-like channels in the whole cell configuration (cv. Franklin, capacitance, 9 pF). The voltage pulse protocol is shown at the top. (B) A current-voltage relation derived from the experiment in (A). Inset shows I/V curve for the tail currents (NORC was pre-activated by the voltage pulse to +80 mV, followed by a pulses to test potentials between −100 and +20 mV). Equilibrium potentials for Cl− (ECl) and K+ (EK) are indicated. (C) Comparison of average NORC-like current per protoplast at +60 mV. Data are means +/− SE; number of successful recordings (protoplasts expressing NORC) are indicated for each cultivar. Total numbers of protoplasts tested were 35 for Gairdner, 19 for Franklin (two salt-sensitive cultivars), 30 for ZUG 293 and 23 for CM72 (two salt-tolerant cultivars).
Figure 2.
Plasma membrane transporters controlling cytosolic K+/Na+ in barley root epidermal cell under salt stress. (1) Increase of NaCl in external medium provokes the Na+ influx through NSCC channels causing a depolarization of plasma membrane. (2) Efflux of K+ across suitable K+- permeable channels, NSCC and KORC. (3) Activation of plasma membrane H+-ATPase opposes NaCl-induced membrane depolarization and linked K+ efflux. (4) Proton gradient built by H+-ATPase assists Na+/H+ antiport, further improving cytosolic Na+/K+ ratio.
The relative contribution of K+-selective and non-selective channels towards salt-induced Na+/K+ exchange may be tissue and species-specific. For instance, a type of NSCC (VIC) dominates the conductance in the pea mesophyll membrane, mediating both Na+ influx and K+ efflux.11 Ca2+-sensitive K+ and Na+ permeable NSCCs control the conductance in Arabidopsis root epidermis and leaf mesophyll tissues, under control conditions and at high external Na+.11 In the frame of the model presented in Figure 2, a suppression of NSCC and KORC activity is beneficial to plants under salt stress. Cationic channels are affected (inhibited) by multivalent cations. Natural polyamines are plausible candidates, with the possibility of manipulating their synthesis and degradation by modulation of their respective enzymes expression, in a tissue-specific manner via stress-induced promoters. Spermine synthesis is induced by salt stress. And genetic manipulations, leading to increased accumulation of the higher polyamines, spermidine and spermine, proved to confer salt-resistance in Arabidopsis, tobacco and rice.13 Recent studies have shown that externally applied polyamines inhibit NSCCs and decrease NaCl-induced membrane depolarization and K+ efflux in pea mesophyll.11 They also block Na+-permeable VIC in barley root epidermis, decreasing Na+ content in roots and shoots of salt stressed plants, thus improving K+/Na+ homeostasis.14 Therefore, manipulation of NSCC expression as well as their inhibition by increased polyamine levels may contribute to improving salinity tolerance in barley and other crops.
Acknowledgements
This work is supported by CONACyT grant CB 59007 to I.I.P. and ARC Discovery grant to S.S. Authors are thankful to Dr. Tracey Cuin for critical reading of the manuscript.
Abbreviations
- NORC
non-selective outward-rectifying conductance
- KORC
K+ outward-rectifying conductance
- VIC
voltage independent cation channel
- NSCC
non-selective cation channels
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5429
References
- 1.Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot. 1999;84:123–133. [Google Scholar]
- 2.Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann Bot. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6:441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 4.Garthwaite AJ, von Bothmer R, Colmer TD. Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl− into the shoots. J Exp Bot. 2005;56:2365–2378. doi: 10.1093/jxb/eri229. [DOI] [PubMed] [Google Scholar]
- 5.Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ. 2005;28:1230–1246. [Google Scholar]
- 7.Chen Z, Zhou M, Newman I, Mendham N, Zhang G, Shabala S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct Plant Biol. 2007;34:150–162. doi: 10.1071/FP06237. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou M, Palmgren MG, Newman IA, Shabala S. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt stressed barley. Plant Physiol. 2007;145:1714–1725. doi: 10.1104/pp.107.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegner LH, Raschke K. Ion channels in the xylem parenchyma of barley roots. Plant Physiol. 1994;105:799–813. doi: 10.1104/pp.105.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegner LH, De Boer AH. Properties of two outward-rectifying channels in root xylem parenchyma cells suggest a role in K+ homeostasis and long-distance signaling. Plant Physiol. 1997;115:1707–1719. doi: 10.1104/pp.115.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shabala S, Cuin TA, Pottosin I. Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 2007;581:1993–1999. doi: 10.1016/j.febslet.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006;141:1653–1665. doi: 10.1104/pp.106.082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett. 2006;28:1867–1876. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhao F, Song CP, He J, Zhu H. Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol. 2007;145:1061–1072. doi: 10.1104/pp.107.105882. [DOI] [PMC free article] [PubMed] [Google Scholar]