Abstract
Doping and manipulation are undesirable companions of professional and amateur sport. Numerous adverse analytical findings as well as confessions of athletes have demonstrated the variety of doping agents and methods as well as the inventiveness of cheating sportsmen. Besides ‘conventional’ misuse of drugs such as erythropoietin and insulins, experts fear that therapeutics that are currently undergoing clinical trials might be part of current or future doping regimens, which aim for an increased functionality and performance or organs and tissues. Emerging drugs such as selective androgen receptor modulators (SARMs), hypoxia-inducible factor (HIF) complex stabilizers or modulators of muscle fiber calcium channels are considered relevant for current and future doping controls due to their high potential for misuse in sports.
Key words: sport, doping, mass spectrometry, anabolics, insulin, HIF, S107
Introduction
Doping has been observed and documented in various facets, all of which arose from the desire of athletes to gain the cutting edge advantage over their competitors. The wish to artificially increase athletic performance and to win by all legal, and sometimes illegal, means is not a contemporary issue but was reported also at the time of the ancient Olympic Games where bribery of judges, manipulation of equipment, and special “diets” to enhance endurance and strength were noted.1 However, the constantly increasing knowledge in medicine, physiology and pharmaceutical sciences have provided virtually unlimited possibilities to influence athletic performance, and numerous sportsmen were convicted of drug abuse and manipulation since sports drug testing was officially established in 1967 by the International Olympic Committee (IOC). The list of prohibited substances and methods of doping has been updated on an annual basis, and since 2001, the World Anti-Doping Agency has taken over the responsibility for the world-wide anti-doping fight.2
Anabolic agents, which include anabolic androgenic steroids but also compounds of non-steroidal structure such as the β2-agonist clenbuterol, have been the most frequently detected prohibited compounds in doping control specimens for more than 20 years and still represent one of the most prevalent threats to the integrity of sport. The major goal of their misuse is reportedly the increase of muscle strength and presumably an accelerated recovery from intense exercise in training or competition periods. In addition, the availability of recombinantly prepared peptide hormones such as erythropoietin (EPO), insulins, human growth hormone (hGH), etc., have challenged sports drug testing laboratories, and detection methods were established years after the misuse of these (glycol) proteins had become evident.3–6 The complex task of differentiating endogenously produced peptide hormones from recombinantly prepared analogs necessitated sophisticated strategies, which have been constantly complemented and improved.7 EPO has been misused to increase endurance by stimulating the erythropoiesis and, thus, the oxygen transport capacities of athletes. Insulin and hGH have been administered for their anabolic and anti-catabolic properties, which supposedly improve muscle tissue growth and preservation from degradation as well as accelerated recovery and reloading of muscle glycogen stores.8
In addition to the assumed and proved misuse of these drugs, new substances and methods of doping and manipulation are suspected that aim for the same ‘beneficial’ effects but using different approaches, because attempts using ‘old fashioned’ compounds might result in adverse analytical findings in doping controls. Hence, preventive doping research has been initiated that shall enable an early implementation of new therapeutics that have not (yet) received clinical approval and might enter the pharmaceutical market in months or years.9,10 Development pipelines are frequently scouted by anti-doping researchers for new substances with great potential for misuse in sports, and detection assays will be developed before a widespread abuse is possible. Selected candidates such as new anabolic agents termed selective androgen receptor modulators (SARMs), rapid acting synthetic insulins such as Humalog or Apidra, hypoxia-inducible factor (HIF) complex stabilizers and modulators of muscle fiber calcium channels (e.g., S107) are presented in the following to provide an impression which challenges sports drug testing might have to face now or in the near future.
Selective Androgen Receptor Modulators (SARMs)
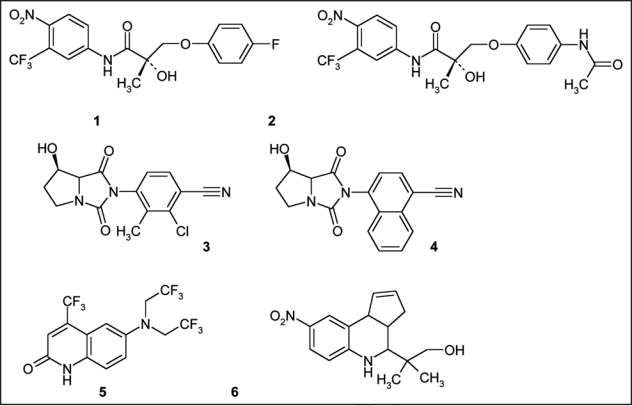
Androgen replacement therapies have been indicated in cases of hypogonadism and wasting diseases; however, common undesirable effects such as benign prostate hyperplasia, acne, various impaired blood parameters and atherosclerosis have necessitated the search alternatives, for instance non-steroidal anabolic agents.11 A first breakthrough was accomplished in 1998, when a SARM based on an arylpropionamide nucleus was synthesized.12 Subsequent structure-activity-relationship studies yielded several lead drug candidates, which are currently undergoing phase II clinical trials, and proof-of-concept study results have shown great promise in terms their clinical utility. The daily administration of 3 mg of Ostarine to a cohort of healthy volunteers over a period of 3 months yielded a highly significant increase of 1.4 kg of lean body mass with improved muscle strength and functionality without applying a particular diet or exercise program.13 Besides, no undesirable effects as commonly found in conventional steroid replacement therapies were observed due to the tissue-selective nature of SARMs. Only androgen receptors in target tissues such as muscles and bones are activated (Fig. 1), while the stimulation of receptors in other, undesired organs such as the prostate, are either not affected or even inhibited. Hence, it is conceivable that these drugs, when legally or even illegally available, will find their way into elite and amateur sport. As a consequence, the World Anti-Doping Agency has added the class of SARMs to the prohibited list in 2008,2 and doping control laboratories have started screening for these drugs. A selection of most advanced SARMs with arylpropionamide-, bicyclic hydantoin-, quinoline- and tetrahydro-quinoline-derived nuclei is illustrated in Figure 2.
Figure 1.
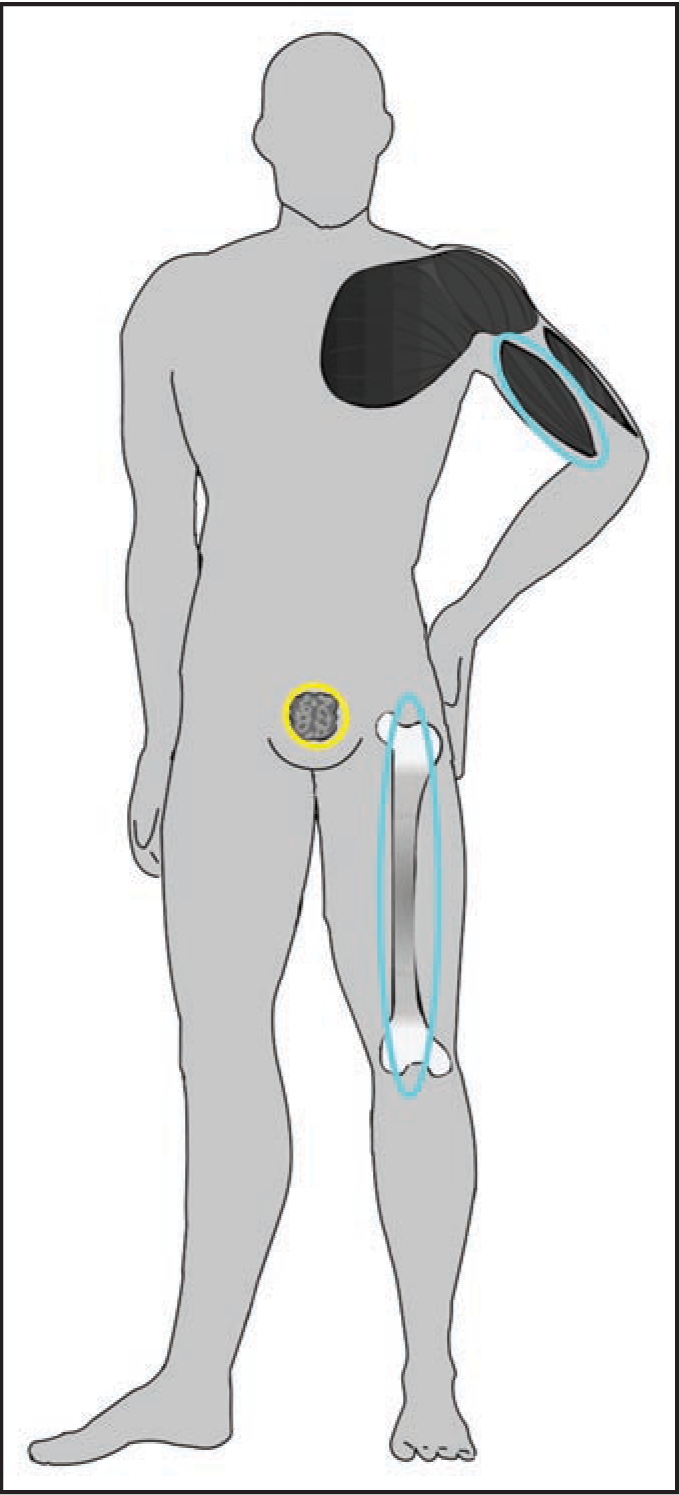
Stimulation (blue) and inhibition (yellow) of androgen receptors accomplished by non-steroidal SARMs. The tissue selectivity and the simultaneously occurring but different effects on distinct organs is of particular importance for steroid replacement therapies.
Figure 2.
Representatives of different classes of SARMs: Arylpropionamide-derived SARMs (Parts 1 and 2), bicyclic hydantoins (Parts 3 and 4), quinoline (Part 5) and tricyclic tetrahydroquinoline (Part 6) derivatives.
Analytically, these compounds represent new classes of targets, which are not covered by a general detection assay due to their diverse physicochemical properties. Arylpropionamide-based SARMs (Fig. 2, Parts 1 and 2) are poorly detected using gas chromatographic (GC) separation before mass spectrometric (MS) analysis, a procedure that is commonly applied to anabolic steroids and respective metabolites. In contrast, liquid chromatography-(tandem) mass spectrometry [LC-MS(/MS)] has demonstrated excellent limits of detection (LODs) for these SARMs, and first approaches targeting the active drugs as well as common metabolites were recently published.14 While arylpropionamide-derived SARMs are sensitively measured using LC-MS/MS,15 bicyclic hydantoin-based analogs (Fig. 2, Parts 3 and 4) are hardly ionized using positive or negative electrospray.16,17 In order to accomplish LODs adequate for an effective anti-doping fight, adduct ion formation using methanol was suggested to provide sufficient amounts of precursor ions for sensitive and selective product ion generation and, thus, detection of these compounds in complex matrices such as urine. In contrast, SARMs with 2-quinolinone nucleus such as LGD-2226 (Fig. 2, Part 5) are measured efficiently using both strategies, GC-MS and LC-MS/MS, due to the considerable volatility of the analyte that is supported by nine fluorine atoms within the molecule.18,19 Most recently, the analysis of a tricyclic tetrahydroquinoline-based SARM (Fig. 2, Part 6) in spiked urine specimens was demonstrated using LC-MS/MS with positive electrospray ionization, which outlines the enormous effort that doping control laboratories have been dedicating to the upcoming issue of SARMs in sport.20 However, all studies also stress the complexity of comprehensively measuring new drugs and possible metabolites, in particular when the metabolic pathways have not been evaluated or published.
Rapid Acting Synthetic Insulins
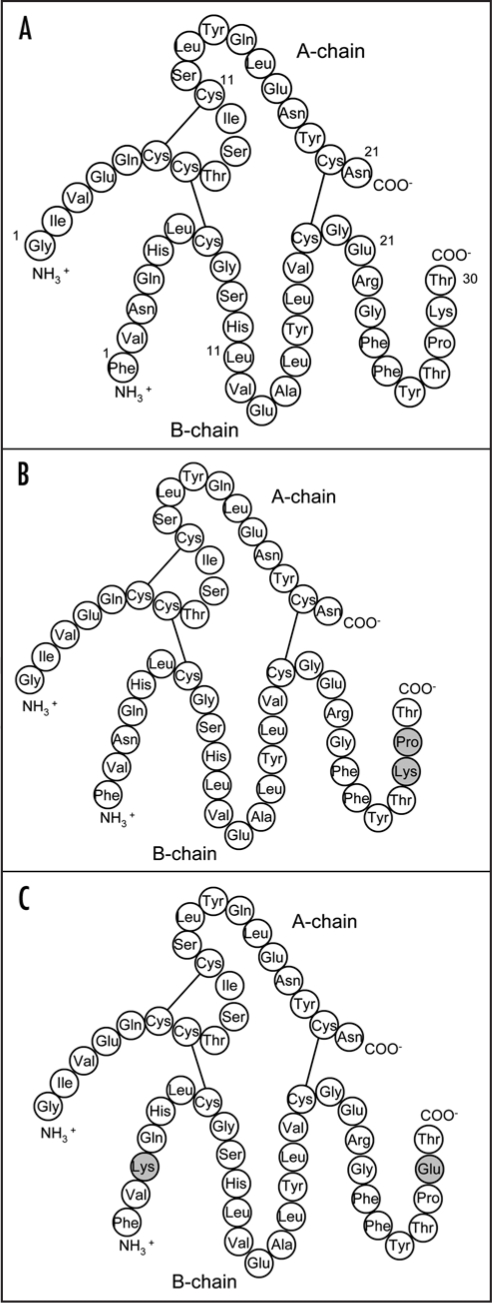
The number of people suffering from diabetes mellitus has been constantly increasing over the last decades, and experts from the International Diabetes Federation expect the disease to become the epidemic of the 21st century.21 Hence, the need for efficient and more convenient drugs for the treatment of diabetes mellitus has prompted pharmaceutical companies to develop rapid- and long-acting synthetic insulins, which differ from human insulin in their primary structure (Fig. 3) and possess different pharmacokinetic properties.22 Typical representatives of rapid-acting synthetic insulins are Humalog LisPro (Fig. 3B) and Glulisine Apidra (Fig. 3C), which are modified compared to human insulin (Fig. 3A) at the amino acid residues B28/B29 and B3/B29, respectively.23 The effect of an altered sequence of amino acid is the reduced aggregation of the bioactive monomers to non-covalent hexamers, which results in a rapid availability of the active drug after subcutaneous injection and, thus, a considerably improved controllability of the therapeutic agent.
Figure 3.
Primary structures of (A) human insulin, (B) Humalog and (C) Apidra. Modifications are highlighted by a grey background.
Insulins have been prohibited in sports for athletes that do not suffer from insulin-dependent diabetes mellitus since 1999. Assumed advantages resulting from the misuse of the peptide hormone are anabolic stimuli, anti-catabolic activities as well as accelerated recoveries from intense exercise by an improved reloading of glycogen stores of muscles.8 In addition to the assumption that athletes might abuse insulins for performance-enhancing properties, confessions of sportsmen, case reports in medical journals as well as popular science literature proved the issue of doping by means of insulin and its synthetic derivatives. Due to the improved ease-of-use of modified insulins, rapid-acting analogs in particular were ‘recommended’ for misuse,24,25 and the complexity to differentiate human insulin from synthetic derivatives was a great challenge for sports drug testing authorities for numerous years, which enabled cheating athletes to use the peptide hormone without getting caught in doping controls. In 2005, a first assay enabling the mass spectrometric detection of synthetic rapid-acting insulins in doping control plasma samples was established based on the fact that modified primary sequences of synthetic insulins either yield different molecular weights or different product ion mass spectra under collision-induced dissociation conditions. Following an immunoaffinity purification step, isolated insulins are chromatographically separated and subjected to tandem mass spectrometry yielding unambiguous results for three different rapid-acting insulin analogs.26 As urine has always been the primary specimen for doping control purposes, the assay was modified in 2006 to allow the detection of insulin derivatives in urine samples. Although the concentration of intact insulin was found to be at least a factor of 10 lower than in plasma specimens, the new approach demonstrated to be an efficient way to identify biotechnologically modified insulins in sports drug testing.27 However, the test has not yet allowed proving the administration of recombinant human insulin to athletes, who are not suffering from diabetes mellitus. Recent preliminary results on the detection of metabolic products of insulins in human urine have shown considerable differences in relative abundances of major metabolites when either administered subcutaneously or produced endogenously and secreted into the blood stream.28 Hence, the evaluation of the renally eliminated metabolites might be an adequate way to determine whether recombinant human insulin is misused by athletes, if future studies will substantiate the preliminary data.
Hypoxia-Inducible Factor Complex Stabilizers
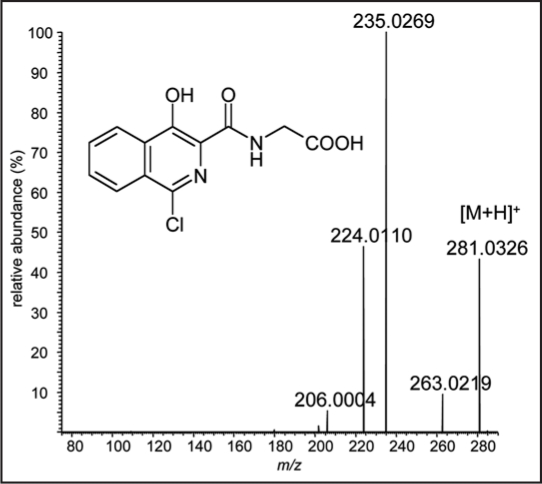
Human oxygen sensing is primarily based on the formation of large protein complexes, which stimulate the production of red blood cells.29–31 In the presence of oxygen, prolyl hydroxylase isoforms catalyze the modification of conserved prolines of two oxygen dependent degradation domains in the hypoxia-inducible factor (HIF)-1α.32–34 The hydroxylation of the proline residues 402 and 564 of HIF-1α represents a ‘label’ for ubiquitylation and subsequent proteasomal destruction.35–37 As a consequence, the presence of HIF-1α in organisms is regulated in an oxygen-dependent manner, which represents the basis of cellular oxygen sensing30,31,38 and, thus, the activity of hypoxic response genes that control angiogenic39,40 and erythropoietic processes.41–43 Based on these findings, new drug candidates were designed and tested regarding their properties to inhibit prolyl hydroxylase activities to counteract and correct symptoms of anemia. One of the most promising classes of candidates, which currently undergoes phase-II clinical trials,44 comprises an isoquinoline core such as the model compound shown in Figure 4. Various studies proved the efficiency of the lead drug candidate FG-2216, an orally active compound in stimulating erythropoiesis, the complete structure of which has not been disclosed yet.45,46 Although not yet commercially available, such substances possess great potential for misuse in sports as conventional drug tests for EPO would fail if HIF complex stabilizers would be applied. In such cases, only endogenously formed EPO would be detected, which does not represent a doping violation; however, the detection of drugs stimulating the EPO production and, thus, artificially increase the amount of red blood cells, is considered a doping offence. EPO has been a serious issue in sports, and numerous confessions of its misuse especially in the 1990s have revealed the dimensions of the former and presumably current prevalence. With the development of detection assays for recombinant EPO, cheating athletes tried to find alternatives, which some of them found in homologous and autologous blood doping.47 Due to the laborious and dangerous nature of blood doping, drugs such as FG-2216 might become subjects of doping attempts and require consideration by doping control authorities. The xenobiotic composition should allow sports drug testing laboratories to detect trace amounts of the active drug or its metabolites in urine specimens as recently demonstrated with a model HIF complex stabilizer, which was analyzed and characterized using high resolution/high accuracy tandem mass spectrometry (Fig. 4).48
Figure 4.
Product ion mass spectrum of the protonated molecule of a model compound representing a new class of HIF-complex stabilizers.
Calstabin-Ryanodine-Receptor Complex Stabilizers
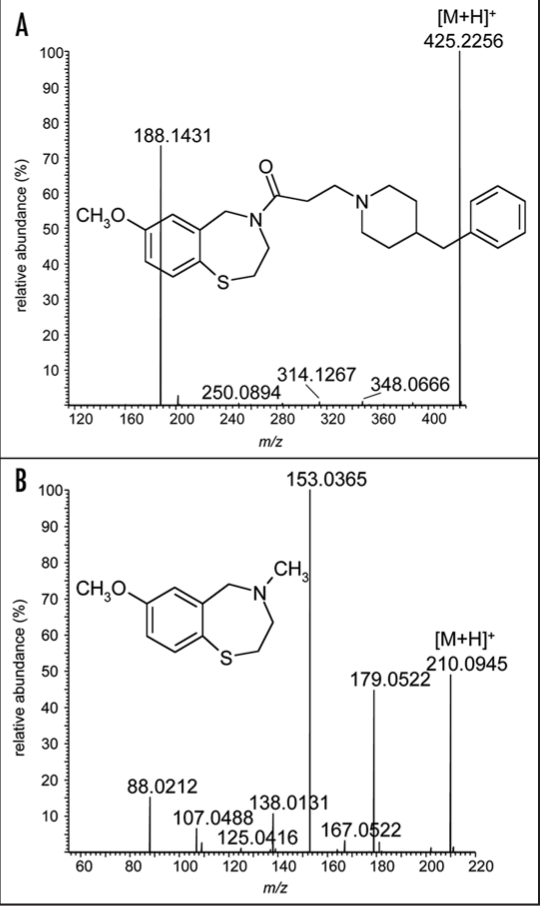
Muscle fatigue can have various reasons, and ventricular arrhythmias resulting in sudden cardiac death have recently been associated with the depletion of the channel-stabilizing protein calstabin2 from the ryanodine receptor-calcium release channel49–54 and the resulting aberrant release of Ca2+ into the cytoplasm. Ca2+ ions are of particular importance for muscle contraction, and intracellular leaks can cause a loss of the depolarization-induced activity. Studies on the influence of low molecular weight compounds based on 1,4-benzothiazepine structures such as JTV 519 (Fig. 5, Part 1) on ventricular arrhythmias have demonstrated great therapeutic utility as these substances increase the affinity of calstabin2 for ryanodine receptor and, thus, stabilize the closed position of the Ca2+ channel. In the course of these investigations, the positive effect of benzothiazepine derivatives on skeletal muscle exercise capacity was observed, which resulted in improved binding of calstabin1 to the skeletal muscle ryanodine receptor (RyR1) and correspondingly in the prevention of “leaky” Ca2+ channels that would entail muscle fatigue.55,56 In addition to JTV 519, a structurally related drug candidate termed S107 (Fig. 5, Part 2) demonstrated great potential in increasing endurance performance as proved with a 3-week daily swimming protocol for mice. Wild-type animals were placed in a pool and induced to swim twice daily, and exercise capacities were assessed once per week by means of treadmill run to exhaustion. A significant increase of the endurance capacity was observed, which allowed the treated mice to run approximately 13 min longer (77.7 ± 4.6 min vs. 64.7 ± 1.4 min) than the control group, which received the vehicle only. The authors concluded that the reduction of exercise-induced Ca2+ leakage by drugs inhibiting the calstabin1 depletion from RyR1 prevents muscle damage, enhances muscle function and exercise capacity.55 Consequently, sports drug testing authorities will have to consider a new dimension of performance manipulation, and detection assays are required that will allow the identification of such drugs in doping control specimens. The structures of the lead drug candidates (Fig. 5) indicate that common drug testing methods will be able to determine the active drug and/or respective metabolites in urine specimens.
Figure 5.
Product ion mass spectra of the drug candidates modulating calcium ion channels in muscle fibers: JTV 519 (A) and S107 (B).
Conclusion
The dynamic nature of drug research and development constantly increases the pool of therapeutics that are potentially misused in sports due to assumed or verified performance enhancing properties. In order to minimize the risk of a head start of cheating athletes that might administer compounds that are still undergoing clinical evaluation, sports drug testing laboratories apply preventive doping research approaches. These imply the monitoring of clinical trials and the implementation of compounds, which are relevant for doping controls, into screening methods as early as possible.
Questions and Answers
Dr. Marc R Hammerman (Chromalloy Professor of Medicine, Washington University School of Medicine): Thank you, Mario, for a very interesting talk. Recently, we have collaborated with you on a project for which you employed mass spectrometry to detect porcine insulin in the circulation of diabetic rhesus macaques that had been transplanted with pig pancreatic tissue.57 Could you comment on the use of techniques you are developing for uses in addition to detection of permormance- enhancing agents?
Dr. Mario Thevis: One of the interesting aspects of our work is the fact that the employed techniques and methods are applicable in various other fields of analytical chemistry. Our collaboration with your group is one of such examples that the sensitive and specific detection and identification of drugs in various biological matrices can be helpful in projects aside sports drug testing. We have been in frequent contact also with forensic scientists and endocrinologists with regard to insulin determinations to provide supporting information in cases of attempted or successful murders. In addition, our analytical approaches were used to characterize growth hormone mutants in studies concerning dwarfism, adaptation processes of tissues, etc.
Dr. Hammerman: Can you provide us with some specific examples (minus names) of how athletes who have used illegal performance enhancing agents have tried to confound the testing procedures?
Dr. Mario Thevis: Various strategies were used to beat the doping control tests, which included for instance urine substitution. Athletes used condoms filled with a clean urine, which were adhered to the skin beyond the scrotum or hidden in the vagina. Using a tiny capillary or simply puncturing the condom caused the clean urine to flow into the collection beaker. Due to various reasons, some of these attempts were revealed, for instance when a male athlete was found ‘pregnant’ because he used his wife's urine. Another group of three athletes provided identical urine specimens, which was uncovered by means of steroid profiling, and DNA comparison of the doping control urine specimens and saliva freshly collected from the athletes demonstrated that none of the sportsmen was the producer of the urine but very likely a relative of one of them. A more recently uncovered strategy to undermine the drug tests was based on proteases. Cyclists in particular confessed that they were using EPO to boost their endurance, but they were never tested positive because they were introducing ‘rice grains’ (protease granules) into their urethra shortly before being guided to the urine collection booth. The protease rapidly dissolved and destroyed all urinary proteins, including the recombinant EPO, which made the test fail due to the absence of any measurable amount of EPO. However, nowadays protease activity and xenobiotic proteases can be detected and identified in urine specimens.
Dr. Hammerman: What has been the most ‘difficult case’ for you to break?
Dr. Mario Thevis: Sports drug testing has been facing numerous challenges, and several still remain unsolved, but we are not the ones to give up. We have been working hard on detection methods for autologous blood transfusions, growth hormones, designer drugs, etc., and in some cases we were successful as recently demonstrated with the finding of 3rd generation EPO during the Tour de France. My personally most interesting and difficult case was the discovery of systematic doping in the Finnish cross-country skiing team in 2001. All team members, which were gold, silver and bronce medal winners at that time, were misusing the plasma volume expander hydroxyethyl starch (HES), and a new procedure that was developed in my group allowed to prove the administration in respective urine specimens. It was a great success and a great catastrophy at the same time as I was an invited external expert supporting the local doping control laboratory in Helsinki (Finland) and, thus, responsible for the most probably biggest doping scandal in Finland ever.
Dr. Sanjay Jain, (Assistant Professor of Medicine and Immunology and Pathology, Washington University School of Medicine): Are drugs used to treat mitochondrial deficiencies used for doping purposes by athletes?
Dr. Thevis: A few drugs that are currently in clinical trials are suspected to be misused, especially PPARdelta receptor activators that are active at the mitochondrial level. There is one particular compound termed GW1516, which has been added to the WADA prohibited list of the forthcoming year. Also here, we are facing a compound that has not entered the pharmaceutical market yet, but there are other approved drugs, especially biguanides such as metformin, which supposedly activate the PPARdelta receptor and are widely available. However, the side effects of these on healthy subjects have been reported unfavourable, and so it remains questionable if these are misused.
Dr. Hammerman: Could you comment about long-term side effects related to the use of doping agents such as anabolic steroids or human growth hormone?
Dr. Thevis: It strongly depends on what kind of drug is misused, also in terms of anabolic androgenic steroids (AAS). AAS can lead to cardiovascular issues such as cardiac hypertrophy, impaired diastolic function and atherosclerosis attributed to decreased serum levels of HDL and increased concentrations of LDL. Prostate growth, acne, and in case of women, virilisation that includes a deeper voice and increased facial hair, were reported. Severe cases were documented regarding female athletes of the former German Democratic Republic (GDR), who converted to male persons. A particular liver toxicity was observed with 17-alkylated steroids such as methyltestosterone and metandienone that reportedly cause benign hepatic adenomas as well as hepatocellular carcinoma. In addition, men frequently suffered from irreversible gynaecomastia as a share of AAS is converted into female sex hormones, and those promote the growth of breasts in men.
Dr. Eduardo Slatopolsky (Joseph Friedman Professor of Medicine, Washington University School of Medicine): What are the levels of circulating testosterone that you measure in athletes who misuse these anabolic agents? Do high levels correlate with more severe side effects?
Dr. Thevis: That is very difficult to answer because the people that have misused AAS are commonly not permanently under clinical observation. We see undesired effects 10 or 15 years later, and then it becomes speculation whether these are all due to the misuse of steroids and, if so, caused by which amounts. In addition, we commonly measure urine samples rather than plasma or blood specimens.
Dr. Slatopolsky: Is this bound or free testosterone that you are measuring?
Dr. Thevis: This is usually total and free testosterone.
Dr. Adriana Dusso (Associate Professor of Medicine, Washington University School of Medicine): Does the use of hypoxia-inducible factor (HIF) complex stabilizers correlate with development of renal carcinoma?
Dr. Thevis: Unfortunately, I can not comment on this question. I looked up clinical trial data for HIF stabilizers, but to my knowledge there is no information on long term consequences available.
Dr. George Jarad (Instructor in Medicine, Washington University School of Medicine): Why is insulin is on the banned substance list?
Dr. Thevis: There are several reasons. Insulin has an anabolic effect, in particular in combination with growth hormone and anabolic steroids, which results in a cocktail that cheating athletes prefer to use, and in addition to that, insulin has proven to accelerate the restoration of glycogen stores. If you are competing each day or even several times a day, an accelerated regeneration is rather important and helpful.
Dr. Jarad: It seems to me that if erythropoietin is a banned substance, the use of a hypoxia chamber should be considered doping, or perhaps even training at high altitudes.
Dr. Thevis: I perfectly agree, and the use of hypoxia chambers has been questioned several times if this is technical doping or not. With regard to high altitude training, we should consider what finally constitutes a doping offense. It is the misuse of drugs and methods. So everything which you can accomplish with natural, normal workout and training is not doping, but the administration of a drug without indication is.
Dr. Hammerman: Could you comment about record-setting performances in decades prior to development of definitive drug testing that haven't been approached since?
Dr. Thevis: A good example how important efficient sports drug testing is, is the comparison of results obtained for instance in 1980 and 2008. The gold medal winner in women's shot put this year in Beijing reached 20.56 m; at the Olympic Games in Moscow 1980, the gold medal winner was able to beat this result by almost 2 m (22.41 m), and it is very likely that this year's Olympic champion would not even have reached the finals. So we can assume that such records will be unbroken forever.
Dr. Hammerman: Does the use of muscle fiber calcium channels modulators ever result in a post-exertional rhabdomyolysis?
Dr. Thevis: That is a good question; of course we do not know anything about the side effects yet, especially not in healthy humans. The studies we know about so far were using rat models but not healthy human elite athletes. But what was demonstrated is that the modifications of rat muscles and human cyclists under exercise conditions were highly comparable, and thus it is conceivable that drugs such as S107 might enable human athletes to compete longer and/or better than usual. However, if signals of fatigue are not sent or recognized anymore, severe health problems and possibly even more serious consequences are possible.
Acknowledgements
The authors thank the Federal Ministry of the Interior of Germany and the Manfred-Donike-Institute for Doping Analysis, Cologne, for supporting the presented work.
Note: Edited transcripts of research conferences sponsored by Organogenesis and the Washington University George M. O'Brien Center for Kidney Disease Research (P30 DK079333) are published in Organogenesis. These conferences cover organogenesis in all multicellular organisms including research into tissue engineering, artificial organs and organ substitutes and are participated in by faculty at Washington University School of Medicine, St. Louis Missouri USA.
Abbreviations
- EPO
erythropoietin
- GC
gas chromatography
- hGH
human growth hormone
- HIF
hypoxia-inducible factor
- IOC
international olympic committee
- LC-MS/MS
liquid chromatography—tandem mass spectrometry
- LODs
limits of detection
- MS
mass spectrometry
- RyR1
skeletal muscle ryanodine receptor
- SARMs
selective androgen receptor modulators
- WADA
world anti-doping agency
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/article/7286
References
- 1.Toohey K, Veal AJ. The Olympic Games—A social science perspective. Oxon—New York: CABI Publishing; 2000. [Google Scholar]
- 2.World Anti-Doping Agency, author. The 2008 Prohibited List. 2008. www.wada-ama.org/rtecontent/document/2008_List_En.pdf.
- 3.Lasne F, Martin L, Crepin N, de Ceaurriz J. Detection of isoelectric profiles of erythropoietin in urine: differentiation of natural and administered recombinant hormones. Anal Biochem. 2002;311:119–126. doi: 10.1016/s0003-2697(02)00407-4. [DOI] [PubMed] [Google Scholar]
- 4.Lasne F, de Ceaurriz J. Recombinant erythropoietin in urine. Nature. 2000;405:635. doi: 10.1038/35015164. [DOI] [PubMed] [Google Scholar]
- 5.Thevis M, Schänzer W. Identification and Characterization of Peptides and Proteins in Doping Control Analysis. Curr Proteomics. 2005;2:191–208. [Google Scholar]
- 6.Pascual J, Belalcazar V, de Bolos C, Gutierrez R, Llop E, Segura J. Recombinant erythropoietin and analogues: a challenge for doping control. Ther Drug Monit. 2004;26:175–179. doi: 10.1097/00007691-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Kohler M, Ayotte C, Desharnais P, Flenker U, Lüdke S, Thevis M, Völker-Schänzer E, Schänzer W. Discrimination of recombinant and endogenous urinary erythropoietin by calculating relative mobility values from SDS gels. Int J Sports Med. 2008;29:1–6. doi: 10.1055/s-2007-989369. [DOI] [PubMed] [Google Scholar]
- 8.Holt RI, Sonksen PH. Growth hormone, IGF-I and insulin and their abuse in sport. Br J Pharmacol. 2008;154:542–556. doi: 10.1038/bjp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thevis M, Geyer H, Mareck U, Schänzer W. Screening for unknown synthetic steroids in human urine by liquid chromatography-tandem mass spectrometry. J Mass Spectrom. 2005;40:955–962. doi: 10.1002/jms.873. [DOI] [PubMed] [Google Scholar]
- 10.Thevis M, Schänzer W. Emerging Drugs—Potential for misuse in sport and doping control detection strategies. Mini-Rev Med Chem. 2007;7:533–539. doi: 10.2174/138955707780619590. [DOI] [PubMed] [Google Scholar]
- 11.Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, Montori VM, Gao W, Dalton JT. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2:146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD. Discovery of nonsteroidal androgens. Biochem Biophys Res Commun. 1998;244:1–4. doi: 10.1006/bbrc.1998.8209. [DOI] [PubMed] [Google Scholar]
- 13.GTx, Inc., author Ostarine achieved the primary endpoint of increasing lean body mass and a secondary endpoint of improving functional performance. 2006. www.salesandmarketingnetwork.com/news_release.php?ID=2015328.
- 14.Thevis M, Schänzer W. Mass spectrometry of selective androgen receptor modulators. J Mass Spectrom. 2008;43:865–876. doi: 10.1002/jms.1438. [DOI] [PubMed] [Google Scholar]
- 15.Thevis M, Kamber M, Schänzer W. Screening for metabolically stable aryl-propionamide-derived selective androgen receptor modulators for doping control purposes. Rapid Commun Mass Spectrom. 2006;20:870–876. doi: 10.1002/rcm.2389. [DOI] [PubMed] [Google Scholar]
- 16.Thevis M, Kohler M, Schlörer N, Kamber M, Kuhn A, Linscheid MW, Schänzer W. Mass spectrometry of hydantoin-derived selective androgen receptor modulators. J Mass Spectrom. 2008;43:639–650. doi: 10.1002/jms.1364. [DOI] [PubMed] [Google Scholar]
- 17.Thevis M, Kohler M, Thomas A, Maurer J, Schlörer N, Kamber M, Schänzer W. Determination of benzimidazole- and bicyclic hydantoin-derived selective androgen receptor antagonists and agonists in human urine using LC-MS/MS. Anal Bioanal Chem. 2008;391:251–261. doi: 10.1007/s00216-008-1882-6. [DOI] [PubMed] [Google Scholar]
- 18.Thevis M, Kohler M, Maurer J, Schlörer N, Kamber M, Schänzer W. Screening for 2-quinolinone-derived selective androgen receptor agonists in doping control analysis. Rapid Commun Mass Spectrom. 2007;21:3477–3486. doi: 10.1002/rcm.3247. [DOI] [PubMed] [Google Scholar]
- 19.Thevis M, Kohler M, Schlörer N, Fusshöller G, Schänzer W. Detection of 2-quinolinone-derived SARMs using GC-MS and LC-MS/MS. In: Schänzer W, Geyer H, Gotzmann A, Mareck U, editors. Recent Advances in Doping Analysis. Cologne: Sport&Buch Strauss; 2008. [Google Scholar]
- 20.Thevis M, Kohler M, Thomas A, Schlörer N, Schänzer W. Doping control analysis of tricyclic tetrahydroquinoline-derived selective androgen receptor modulators using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:2471–2478. doi: 10.1002/rcm.3637. [DOI] [PubMed] [Google Scholar]
- 21.International Diabetes Federation, author. Diabetes epidemic out of control. 2006. www.idf.org/home/index.cfm?unode=7F22F450-B1ED-43BB-A57C-B975D16A812D.
- 22.Barnett AH, Owens DR. Insulin analogues. Lancet. 1997;349:47–51. doi: 10.1016/S0140-6736(96)06032-1. [DOI] [PubMed] [Google Scholar]
- 23.Thevis M, Thomas A, Schänzer W. Mass spectrometric determination of insulins and their degradation products in sports drug testing. Mass Spectrom Rev. 2008;27:35–50. doi: 10.1002/mas.20154. [DOI] [PubMed] [Google Scholar]
- 24.Conte V. Times Online (London) 2008. Victor Conte's letter to Dwain Chambers. [Google Scholar]
- 25.Steiner A, Wagner RA. Insulin. Vol. 3. Arnsberg: ISP-Verlag; 2002. pp. 4–73. [Google Scholar]
- 26.Thevis M, Thomas A, Delahaut P, Bosseloir A, Schänzer W. Qualitative Determination of Synthetic Analogues of Insulin in Human Plasma by Immunoaffinity Purification and Liquid Chromatography-Tandem Mass Spectrometry for Doping Control Purposes. Anal Chem. 2005;77:3579–3585. doi: 10.1021/ac050066i. [DOI] [PubMed] [Google Scholar]
- 27.Thevis M, Thomas A, Delahaut P, Bosseloir A, Schanzer W. Doping control analysis of intact rapid-acting insulin analogues in human urine by liquid chromatography-tandem mass spectrometry. Anal Chem. 2006;78:1897–1903. doi: 10.1021/ac052095z. [DOI] [PubMed] [Google Scholar]
- 28.Thomas A, Thevis M, Delahaut P, Bosseloir A, Schanzer W. Mass spectrometric identification of degradation products of insulin and its long-acting analogues in human urine for doping control purposes. Anal Chem. 2007;79:2518–2524. doi: 10.1021/ac062037t. [DOI] [PubMed] [Google Scholar]
- 29.Bruick RK, McKnight SL. Transcription. Oxygen sensing gets a second wind. Science. 2002;295:807–808. doi: 10.1126/science.1069825. [DOI] [PubMed] [Google Scholar]
- 30.Bruick R. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 31.Safran M, Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsilä M, Koivunen P, Günzler V, Kivirikko K, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 33.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 34.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 35.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 36.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 37.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzen E, Ratcliffe P. HIF hydroxylation and cellular oxygen sensing. Biol Chem. 2004;385:223–330. doi: 10.1515/BC.2004.016. [DOI] [PubMed] [Google Scholar]
- 39.Warnecke C, Griethe W, Weidemann A, Jurgensen J, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei U, Wiesener M, Eckardt K. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J. 2003;17:1186–1188. doi: 10.1096/fj.02-1062fje. [DOI] [PubMed] [Google Scholar]
- 40.Bruick RK, McKnight SL. Building better vasculature. Genes Dev. 2001;15:2497–2502. doi: 10.1101/gad.931601. [DOI] [PubMed] [Google Scholar]
- 41.Jelkmann W. Molecular biology of erythropoietin. Intern Med. 2004;43:649–659. doi: 10.2169/internalmedicine.43.649. [DOI] [PubMed] [Google Scholar]
- 42.Ratcliffe P. From erythropoietin to oxygen: hypoxia-inducible factor hydroxylases and the hypoxia signal pathway. Blood Purif. 2002;20:445–450. doi: 10.1159/000065201. [DOI] [PubMed] [Google Scholar]
- 43.del Peso L, Castellanos M, Temes E, Martin-Puig S, Cuevas Y, Olmos G, Landazuri M. The von Hippel Lindau/hypoxia-inducible factor (HIF) pathway regulates the transcription of the HIF-proline hydroxylase genes in response to low oxygen. J Biol Chem. 2003;278:48690–48695. doi: 10.1074/jbc.M308862200. [DOI] [PubMed] [Google Scholar]
- 44.Fibrogen Inc., author Development Pipeline. 2007. http://www.fibrogen.com/pipeline/chart/anemia.html#mid.
- 45.Wang Q, Gou G, Guenzler V, Neff T, Klaus S, Turtle E, Molineaux C, Yeowell D, Lin A. Stimulation of erythropoiesis and treatment of anemia in rodents by oral administration of FG-2216, a novel HIF-prolyl hydroxylase inhibitor. J Am Soc Nephrol. 2004;15:773. [Google Scholar]
- 46.Hsieh MM, Linde NS, Wynter A, Metzger M, Wong C, Langsetmo I, Lin A, Smith R, Rodgers GP, Donahue RE, Klaus SJ, Tisdale JF. HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–2147. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stern, author. Inside the Blood Doping Investigation. 2006. www.spiegel.de/international/spiegel/0,1518,425939,00.html.
- 48.Thevis M, Kohler M, Schlörer N, Schänzer W. Gas Phase Reaction of Substituted Isoquinolines to Carboxylic Acids in Ion Trap and Triple Quadrupole Mass Spectrometers after Electrospray Ionization and Collision-Induced Dissociation. J Am Soc Mass Spectrom. 2008;19:151–158. doi: 10.1016/j.jasms.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 50.Wehrens XH, Marks AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem Sci. 2003;28:671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 52.Lehnart SE, Wehrens XH, Marks AR. Calstabin deficiency, ryanodine receptors and sudden cardiac death. Biochem Biophys Res Commun. 2004;322:1267–1279. doi: 10.1016/j.bbrc.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 53.Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- 54.Wehrens XH, Lehnart SE, Marks AR. Ryanodine receptor-targeted anti-arrhythmic therapy. Ann NY Acad Sci. 2005;1047:366–375. doi: 10.1196/annals.1341.032. [DOI] [PubMed] [Google Scholar]
- 55.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellinger AM, Mongillo M, Marks AR. Stressed out: the skeletal muscle ryanodine receptor as a target of stress. J Clin Invest. 2008;118:445–453. doi: 10.1172/JCI34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers SA, Chen F, Talcott MR, Faulkner C, Thomas JM, Thevis M, Hammerman MR. Long-term engraftment following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic rhesus macaques. Xenotransplantation. 2007;14:591–602. doi: 10.1111/j.1399-3089.2007.00429.x. [DOI] [PubMed] [Google Scholar]