Abstract
Flower senescence is the terminal phase of developmental processes that lead to the death of flower, which include, flower wilting, shedding of flower parts and fading of blossoms. Since it is a rapid process as compared to the senescence of other parts of the plant it therefore provides excellent model system for the study of senescence. During flower senescence, developmental and environmental stimuli enhance the upregulation of catabolic processes causing breakdown and remobilization of cellular constituents. Ethylene is well known to play regulatory role in ethylene-sensitive flowers while in ethylene-insensitive flowers abscisic acid (ABA) is thought to be primary regulator. Subsequent to perception of flower senescence signal, death of petals is accompanied by the loss of membrane permeability, increase in oxidative and decreased level of protective enzymes. The last stages of senescence involve the loss of of nucleic acids (DNA and RNA), proteins and organelles, which is achieved by activation of several nucleases, proteases and wall modifiers. Environmental stimuli such as pollination, drought and other stresses also affect senescence by hormonal imbalance. In this article we have covered the following: perception mechanism and specificity of flower senescence, flower senescence-associated events, like degradation of cell membranes, proteins and nucleic acids, environmental/external factors affecting senescence, like pollination and abiotic stress, hormonal and non-hormonal regulation of flower/petal senescence and finally the senescence associated genes (SAGs) have also been described.
Key Words: environmental factors, ethylene, flowers, petals, plant hormones, pollination, programmed cell death, senescence, senescence-associated genes
Introduction
Plant senescence is a developmentally regulated and genetically programmed process that ultimately leads to death of a particular organ or whole plant. It is an important and final event of plant development and usually observed in many different tissues such as leaves, petals, reproductive organs (stamens and style), root cap, cortex, germinating seeds etc. Flower parts provide an excellent model for both the abscission and senescence because both processes are irreversible and are under precise developmental control. The senescence event involves structural, biochemical and molecular changes, which are also the hallmarks of programmed cell death (PCD). Generally in plants the term senescence and PCD both denote the processes that initiate the programmed death of individual cells.1 Therefore the senescence can also be considered as one of the examples for PCD.
Senescence is a highly controlled process and involves the progressive shut down of several biosynthetic pathways and the expression of different hydrolases that hydrolyse polymers such as carbohydrates, proteins, lipids and nucleic acids. The hydrolyzed products are then transported to newly growing tissues where they are needed. Thus plant senescence is a vital process that saves energy and materials with recycling program. There is a complex interplay of different hormones during the senescence, for example ethylene and ABA play a major role in the onset of senescence while cytokinins prevent it. The mechanism by which the hormones or their precursors are rapidly transmitted and sensed to bring about the changes in different tissues such as petals, stamens and ovary are not yet well known.2
Flower Senescence: Perception Mechanism and Specificity
Flowers play very critical role in plants' life cycle. These are structures responsible for sexual reproduction and seed set for continuation of one generation to next generation. Each flower parts have to play a definite role and are only retained when needed. So it becomes important for the plant to strictly regulate the death of its flower.3 Most of the changes in flowers is a consequence of pollination. Pollination is known to trigger a large number of changes in flowers such as petal and anther senescence/abscission, and ovary maturation to contain developing seeds.4 In several species like Petunia, tobacco and carnation, flower senescence is mediated by pollination-induced ethylene generation.4
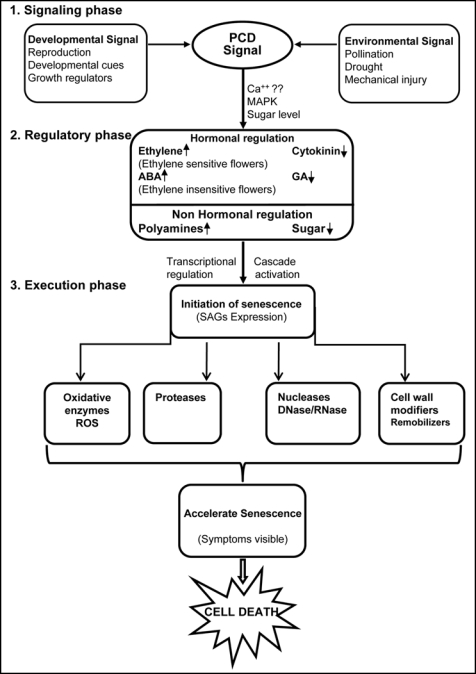
A model for regulatory pathways in flower senescence is depicted in Figure 1. ten Have and Woltering (1997)5 showed that the pollination-induced ethylene in carnation is translocated from stigma to the petals via the style and ovary, where it upregulates ethylene biosynthetic genes and induces the production of ethylene. Once the process is initiated the evolution of ethylene becomes autocatalytic and accelerates the senescence process.6 This suggests that the promoters of ethylene biosynthetic genes contain some ethylene-responsive elements.2 In several ethylene signalling mutants in Arabidopsis, like etr1, ein2, ein3 and ers2 that are affected in ethylene perception, senescence of floral organs is delayed but not blocked. In these mutants expression of senescence-associated genes (SAGs) was also delayed temporally but the expression level was similar to wild-type plants (Fig. 1). This led to the conclusion that ethylene is involved in regulation and control of the timing of floral organ senescence, but not required for the execution of the senescence program once it had begun. This suggests that rather than ethylene some other ethylene-independent pathways are also required for initiation and progression of senescence.7
Figure 1.
A model for regulatory pathways in flower senescence. The PCD signal is generated by both external and internal stimuli and transduced by some signals resulting hormonal imbalance in the cell. This altered level of hormones further activates several cascade and transcriptional regulation. The initiation of senescence starts with expression of several SAGs like proteases, nucleases, wall degrading and oxidative enzymes. Now the collective actions of all these enzymes accelerate the senescence process and it become irreversible. At the later stage of senescence symptoms become visible and ultimately leads to cell death of flowers.
Based on ethylene sensitivity flower senescence can be divided into two groups.8 One, with ethylene as the major regulator, i.e., ethylene-dependent pathway as in Petunia, carnation, Ipomoea, rose, Arabidopsis etc., and the other ethylene-independent pathway as in Alstroemeria, Gladiolus, Iris, Sandersonia and daylily. Several studies have shown the ethylene-independent pathway regulating floral degeneration,9,10 but their mechanism is not yet well studied. Thomas et al. (2003)11 proposed that senescence/PCD is regulated at both the transcriptional (initiation and activation of SAGs) and the post-transcriptional level (execution and fine control). Thus the ethylene-sensitive and ethylene-insensitive flower senescence processes may have some common cellular components with complex network of post-transcriptional modifications. Langston et al. (2005)12 reported that nuclear DNA fragmentation and the induction of a nuclease (PhNUC1) were delayed but not eliminated during flower senescence in ethylene-insensitive, 35S:etr1-1 transgenic Petunias. However it is still unclear whether there are components of the senescence pathways in petals that are dependent on ethylene.
Since each part of flower play different role in their life span, therefore it requires specific co-ordination of sencence signal among them. Rieu et al. (2003)13 showed that ethylene regulates petal senescence in tobacco and at the same time ovary continues to develop without any interruption. Wagstaff et al. (2003)14 revealed that petal margins degenerate before the centre, suggesting existence of the gradient of signal or receptor or other mediators of PCD. Christensen et al. (2002)15 reported that in Arabidopsis gfa2 mutant flowers synergids failed to undergo PCD while antipodal PCD was not affected, which indicated that the specificity of signal is required for the regulation of specific cellular target of PCD.
Based on the PCD mechanism established in morphological types of metazoan cell death, van Doorn and Woltering (2005)16 tried to categorize plant PCD in three types: apoptotic-like, autophagic and neither apoptotic nor autophagic. Similar to animal apoptosis, tapetum and pollen tube showed clear evidence to support an important role for the mitochondrion and involvement of caspases.16 Tapetum degeneration is characterized by chromatin condensation in Lobivia and Tillandsia flowers,17 and by DNA fragmentation in barley anthers.18 In sunflower anthers cytoplasmic male sterility (CMS) there is a cytochrome c release into the cytosol followed by changes in the cell morphology and loss of outer mitochondrial membrane integrity.19 However, PCD mechanism in the tapetum is still unknown. It has been shown that self-incompatibility also triggers PCD in Papaver pollen tube by induced caspase-3-like activity.20 Recently, Thomas et al. (2006)21 established that changes in actin filament levels or dynamics play a functional role in initiating PCD in Papaver pollen, triggering a caspase-3-like activity thus providing a link between actin polymerization and PCD in pollen.
In autophagy, the type of PCD in petals is characterized by the identification of vesicles that transported proteases to the vacuole. The endoplasmic reticulum-derived compartments contain precursors of cysteine proteinases known as “protease precursor vesicles” (PPVs), which deliver vacuolar-processing enzymes (VPE) to the vacuole where they are required for the processing of a number of proteins.22 Several proteases have been identified in a number of senescent floral organs of Petunia,23 daylily petals24 and in pea ovaries.25 The third category of PCD is not very well characterized in flowers.
Sepal senescence shows similarity with leaf senescence because they are green and contains chlorophyll. In broccoli flower, sepal chlorophyll degradation is the first visual sign of senescence.26
Flower Senescence Associated Events
The developmental events taking place during senescence also involve physiological changes such as loss of water from the senescing tissue, leakage of ions, transport of metabolites to different tissues, and biochemical changes, such as generation of reactive oxygen species (ROS), increase in membrane fluidity and peroxidation, hydrolysis of proteins, nucleic acids, lipids and carbohydrates.
Cell membrane degradation.
Membrane deterioration is an early and characteristic feature of plant senescence, which leads to several structural and functional changes. For example the ultra-structural changes including the vesiculation of vacuolar and cytosolic compartments have been reported in carnation petals.27 In daylily flowers, during senescence the degeneration of vacuolar membrane of epidermal cells was reported by Stead and van Doorn (1994).28
At the biochemical level, senescence is associated with changes in membrane fluidity and leakage of ions in several different flowers.29 The loss of differential membrane permeability was reported by Phillips and Kende (1980)30 in morning glory flower. The important changes at the membrane, which include the decrease in all classes of membrane phospholipids and increase in neutral lipids, mainly due to increased action of phospholipases and acyl hydrolases, have been reported.31 Another important event that leads to loss of membrane permeability is oxidation of membrane lipids (lipid peroxidation) due to lipoxygenases in day lily32 and carnations.33 Hong et al. (2000)34 showed that an ethylene-inducible lipase mRNA increases just as carnation flowers begin to senesce. Leverentz et al. (2002)35 revealed that in ethylene-insensitive Alstroemeria flower senescence the loss of membrane function was not related to lipoxygenase activity. In the ethylene-sensitive category, lipoxygenase activity may promote senescence through oxidative membrane damage as seen in rose36 and carnation.37 However, in some ethylene-sensitive plants such as Phalaenopsis, the lipoxygenase seems not to play any apparent role.38 On the other hand, in the ethylene-insensitive category, lipoxygenase promotion of senescence has been proposed in daylily39 and implicated in Gladiolus species.40 Thus Alstroemeria represents a distinct pattern of floral senescence that is both ethylene- and lipoxygenase-independent.
Due to various oxidative reactions, ROS are known to be involved in normal death of plant cells including petals. The ROS is produced from hydrogen peroxide, thus the hydrogen peroxide level regulating enzymes showed differential expression during senescence.41 In daylily, an increase in ROS due to a decrease in activity of catalase and increased activity of superoxide dismutase (SOD) was observed.32 In carnation petals catalase and ascorbate peroxidise (APX) activities increase during flower senescence.42 An increase in the number of peroxisomes during carnation petal senescence has also been reported.43 Decreased levels of natural antioxidants were reported in several flowers.44 During the flower senescence membrane proteins mediating transport (various cellular pumps) and redox reactions were reported to decrease.45
Protein degradation: Proteases in flower/petal senescence.
During the time course of senescence targeted protein degradation is a critical part, therefore the activity of proteolytic enzymes is an essential element in these processes. Proteases degrade proteins by hydrolyzing internal peptide bonds and are one of the best characterized cell death proteins in plants.46 PCD-promoting signals induce inactive zymogens to active proteases and trigger irreversible proteolysis cascade causing death. Among all the proteases, the cysteine proteases are the most frequent and well characterized.47 In support of their role in flower senescence several cysteine proteases have been shown to be upregulated and further cloned from petals of Petunia,23 Alstroemeria,48 Sandersonia,49 and Narcissus.50
Using differential screening of a cDNA library, Guerrero et al. (1998)51 reported a cDNA clone encoding a daylily thiol protease (SEN11), whose expression is strongly upregulated in flower tepal senescence. Earlier reported thiol protease SEN10224 and SEN11 transcripts were not detectable in flower buds at the opening stage, but a significant increase in transcripts in both wilting petals and sepals was reported. The expression pattern of these genes coding for proteases suggests their involvement in the protein hydrolysis in tepals at the late senescence stage. Cercós et al. (1999)25 showed the elevated expression of a thiol-protease TPE4A during senescence of unpollinated pea ovaries. Recently Azeez et al. (2007)52 reported the expression of specific serine proteases during senescence-associated proteolysis in Gladiolus flowers. During the senescence, serine protease activity increases up to 2/3 of total proteases activity. In-gel assay analysis revealed the enhanced activity of two trypsin-type serine proteases with different molecular masses (75–125 kDa) during the course of senescence.
Several senescence-associated cysteine protease genes have been reported to increase in abundance following ethylene treatment. In Petunia hybrida a total of nine cysteine protease genes were analyzed in ethylene-sensitive corollas and six of the nine cysteine proteases showed increased transcript abundance during ethylene-induced petal senescence.23 In carnation the expression of cysteine protease DCCP1 increased several-fold after three hours of ethylene treatment during flower petal senescence.53 Sugawara et al. (2002)54 cloned a gene from carnation flower for the cysteine protease inhibitor that is expressed abundantly in the petals at the full opening stage of the flower and during senescence its expression declines temporally. This inhibitor may be considered to play a role in the regulation of petal senescence by fine tuning the expression of different cysteine proteases.
Nucleic acid degradation.
During senescence of floral parts, the degradation of DNA and RNA is the most common feature.11 Using differential screening, Panavas et al. (1999)55 cloned a cDNA from daylily petals, with similarity to fungal S1- and P-type endonucleases that degrade both single-stranded DNA and RNA and only a single copy of the petal-specific gene was reported. The transcript level of this putative nuclease increases at flower opening and continues to increase during senescence. The advanced stages of petal senescence in Petunia were found to be associated with DNA laddering and increased nuclease activity.56 Five DNases with specific activity against ssDNA were identified in petals of Petunia and all of them were shown to be increased during the senescence of pollinated flowers. Langston et al. (2005),12 using the same flower species, characterized a single cobalt-dependent senescence-specific nuclease PhNUC1, which is a glycoprotein and is characterized by higher activity during both pollination-induced and age-related senescence. Activity of PhNUC1 was induced in non-senescing corollas by treatment with ethylene. The increased PhNUC1 activity was delayed in ethylene-insensitive flowers (35S:etr1-1) suggesting ethylene-dependent regulation of DNA fragmentation and nuclease activity. In another study using flow cytometry and fluorescence microscopy, Yamada et al. (2006)57 reported DNA degradation, chromatin condensation and nuclear fragmentation during PCD in petals of Ipomoea. Senescence-associated RNases have also been characterized from petals of Arabidopsis58 and tomato.59
Recently, Yamada et al. (2006)60 studied flower PCD in the petals of Antirrhinum, Argyranthemum and Petunia, using DNA degradation and changes in nuclear morphology as parameters. The petals of all three flowers showed loss of turgor (wilting) and DNA degradation. Two distinct types of nuclear morphology were observed during PCD in these petals. One type was characterized by chromatin clumping into spherical bodies inside the nuclear membrane lacking nuclear fragmentation during PCD as in Antirrhinum. Nuclear fragmentation did not occur in Antirrhinum, whereas nuclear fragmentation was reported in Argyranthemum and Petunia.60
Environmental Factors Affecting Senescence
Senescence might be triggered by biotic and abiotic stresses. Flowers being most colorful and susceptible part of plant are easily affected by several environmental factors such as insect-mediated pollination, seasonal changes, lack of water, and various stresses such as invasion by a pathogen or attack by a predator.61
Pollination.
It is now well established that compatible pollination activates a series of post-pollination developmental events contributing to reproduction and ovary growth, pigmentation changes and petal senescence.62,63 The post-pollination events trigger increase in hydrolytic enzymes and degradation of macromolecules. Pollination-induced senescence is accelerated in 60 different genera, most of which are suspected of being sensitive to ethylene.64
Pollination-regulated development is initiated at the stigma but the developmental processes, like petal senescence, take place in floral organs.2 These observations suggest that there are some inter-organ signals that amplify and transmit the pollination signal to floral organs. It is reported that inter-organ signalling and signal amplification involves the regulation of ethylene biosynthetic gene expression and inter-organ transport of hormones and their precursors.62 In carnation petals, the signal arises from the compatible reaction between pollen and stigma.65 In this flower, a burst of ethylene occurs apart from whether the pollen is compatible or incompatible, which may be due to the presence of endogenous ACC in the pollen grain. Senescence, however, occurs after a second burst of ethylene that is reported only when compatible pollination took place.66 An ACC synthase gene was reported to be upregulated by pollination and auxin in the stigma.67 In contrast to carnation, in Petunia styles the ACC upregulation and transportation were not reported.68 There is also an ethylene-independent increase in sensitivity to the hormone after pollination, apart from an increase in ethylene production.69 The role of short-chain saturated fatty acids in the control of ethylene sensitivity in senescing carnation has been reported.70
Abiotic stresses.
Flower senescence is often hastened by several abiotic stresses, like drought, light quality, heat shock etc. In these stresses the senescence process is mediated by the evolution of ethylene in ethylene-sensitive flowers, while in ethylene-insensitive flowers elevated level of ABA might be an important hormonal intermediate signal for responses. Panavas et al. (1998)32 reported that drought stress leads to early senescence symptoms in sorbitol-treated daylily flowers. Elevated ABA level was observed in drought-induced senescence of daffodil,71 carnation72 and daylily.32 A change in light source also significantly affected the ABA content of the petal. Garello et al. (1995)73 showed that rose flowers under sodium lamps showed delayed senescence as compared to metal halide lamps. There is an inverse relationship between ABA level and flower longevity as the higher ABA level the shorter the vase-life observed. Woltering et al. (1997)74 reported higher ethylene level in excised gynoecium in the presence of light than dark. Under the heat shock, Verling et al. (1991)75 reported the upregulation of heat shock proteins and downregulation of several other proteins. There are several reports that the heat shock delays the senescence related genes up to considerable time period in several flowers like carnation76 and daylily.32 This may be due to strong activation of some defence related genes which mimic the expression of PCD genes.
Hormonal Regulation of Flower/Petal Senescence
Several plant hormones were reported to play an important role in senescence process. The specific regulation of flower senescence is very complex process and is governed by endogenous levels and sensitivity of hormones. It is difficult to say that all the hormones are actively involved in floral PCD in all species. There should be some quantitative and qualitative species-specific differences present to mediate programmed cell death. In most of the flowers, it is an increase in ethylene following pollination that triggers the changes associated with petal senescence. The roles of individual hormones are discussed below.
Ethylene.
Senescence in many plants is accelerated by the naturally occurring plant hormone ethylene. Ethylene is a gas produced by plants and exposure to ethylene causes premature senescence of both the leaves and flowers. In flowers, senescence is triggered by hormonal changes that follow pollination. Like fruits, most flowers also show a climacteric rise in ethylene following pollination.4 In flowers like Petunia that are self-incompatible, the senescence-related changes in petals are observed after compatible pollination and not after pollination by incompatible pollen.66
The role of ethylene in flower development has been studied to a great extent in Petunia, Geranium, orchids, and carnation. In Petunia, out of four ACC oxidase (ACO) gene members ACO1 is expressed specifically in senescing corollas and in other floral organs following ethylene exposure while ACO3 and ACO4 were expressed in developing pistil tissue.77 The timing and tissue specificity of the increased expression of ACO transcipts correlated with pollination-induced ethylene production in styles and stigma followed subsequently by corollas leading to senescence.78 Pollination in orchids was associated with an increase in ACO transcript in stigma and petals.79 Ethylene biosynthetic genes were also differentially expressed during carnation flower senescence.5
Ethylene receptors and downstream genes of ethylene signaling cascade were also shown to play an active role in the progression of senescence. In carnation, Shibuya et al. (2002)80 showed that DC-ERS2 and DC-ETR1 are ethylene receptor genes responsible for ethylene perception and that their expression during flower senescence is regulated in a tissue specific manner and independently of ethylene. In roses, senescence of flowers seems to be related to elevated levels of ETR3 indicating that flower senescence may be triggered by the perception of endogenous ethylene by ethylene receptors.81 Expression of ethylene receptor gene ERS1 and signaling regulator gene CTR1 from Delphinium florets was found to increase after treatment of florets with ethylene.82 In Rosa hybrida higher expression of two CTR genes were reported during ethylene induced flower senescence. RhCTR1 levels were upregulated in senescing flowers, while RhCTR2 was constitutively expressed during flower senescence.83 In Dianthus a putative EIN3-like protein, DC-EIL1 transcript level decreased in flower tissue, especially in petals, during natural senescence and in response to ethylene and ABA treatment.84 Similarly, in rose the gene RhEIN3 was constitutively and stably expressed during flower development in response to ethylene and ABA.85 These findings suggest that control of ethylene sensitivity during senescence occurs upstream of EIN3 and its homologues.
Several ethylene mutants were studied in order to elucidate the role of ethylene in regulation of flower senescence. Transgenic carnation flower with antisense ACO showed delayed senescence which enhanced the shelf life from five days to nine days.86 The expression of the Arabidopsis etr1-1 gene in transgenic carnations Petunia and tomato delayed flower senescence, resulting in a significant increase in vase life.87,88
Abscisic acid.
Exogenous application of ABA to certain flowers hastens flower senescence.89 In daylilies, which are ethylene-insensitive, ABA is thought to be the primary hormonal regulator of flower senescence, and many of the senescence-related changes are brought about by ABA. These include ion leakage, changes in lipid peroxidation, protease activity and expression of novel DNases and RNases.90 In ethylene-sensitive flowers, like carnation, ABA accelerates flower senescence by increasing the endogenous production of ethylene.91 Hunter et al. (2004)92 reported that the ABA content increased in tepals of senescent flowers. The increased ABA content coincided with the appearance of visual signs of senescence in tepals. Exogenous ABA enhanced the premature accumulation of senescence-associated transcripts in the tepals indicating that ABA induced the transcripts independently of ethylene.
Cytokinins.
In contrast to ethylene and ABA, cytokinins delay senescence in floral tissues. The inverse relationship between cytokinin content and senescence occurs in some flowers, like Petunia,93 roses94 and carnation.95
In an interesting study, Chang et al. (2003)96 confirmed the role of cytokinins in flower senescence. The transgenic plants overexpressing IPT gene under the SAG12 promoter exhibited significant delays in flower senescence resulted in the increased cytokinin content and less ethylene sensitivity. Increased endogenous ethylene production was measured after pollination but this increase was delayed in IPT flowers. Flowers from IPT plants were less sensitive to exogenous ethylene, and showed delayed ethylene responsiveness, corolla senescence and a senescence-related cysteine protease. These plants confirm that the regulation of flower senescence involves the interaction of cytokinins, ethylene and ABA.
Auxins, gibberelic acids and jasmonic acid.
The effect of auxins and gibberelic acids is not well characterized in flower senescence. Applications of auxins to cut flowers stimulate senescence of some ethylene-sensitive flowers.62 Gibberellic acid is known to delay senescence in some cut flowers by acting as an antagonistic to ethylene. Saks et al. (1992)97 showed that exogenous application of gibberellic acid delayed the senescence of cut carnation flowers with reduced ethylene production. Recently, Setyadjit et al. (2006)98 treated an ethylene-sensitive flower Grevillea ‘Sylvia’ and this treatment did not enhance the longevity of inflorescences and ACC level. At higher level gibberellic acid concentrations enhanced flower abscission rather than senescence. Jasmonic acid also shows stimulating effect on flower senescence. Jasmonic acid along with several other metabolites promotes senescence of orchid species, presumably by elevating ACC, thereby stimulating ethylene production. However, in orchid petals for 50 h after pollination-induced senescence neither lipoxygenase activity nor jasmonic acid content changed.38
Non-Hormonal Regulation of Flower Senescence
The flower senescence is also known to be regulated by several signaling pathways including G protein and calcium signaling, polyamines and sugars. A heterotrimeric G proteins linked phospholipase a and c (PLA and PLC) increase in petals of roses and Petunia before the onset of senescence.99 PLC is known to hydrolyze the membrane component phosphatydiylinositol diphosphate to yield inositol triphosphate and diacylglycerol (DAG). In addition, DAG level increased before to the burst of ethylene in Petunia flower senescence.100
Secondary messenger calcium levels increase in leaves concomitant with senescence 101 and in the orchid Phalaenopsis, exogenous calcium increased flower sensitivity to applied ethylene accelerating senescence.102 The possibility thus exists that the calcium wave is the signal for onset of senescence. This increased level of calcium may result in the calcium-dependent phosphorylation of proteins that are necessary for the upregulation of suicide proteins.
In plants, polyamines (PAs) have a well established role in the stimulation of cell division, in growth, and in the delay of senescence, hence called as ‘juvenility’ factors. The senescence of carnation petals is inhibited by spermine, which may be due to a corresponding inhibition of ethylene synthesis. Addition of an inhibitor of polyamine synthesis leads to elevated levels of transcripts for ACC synthase and ACC oxidase as well as to increased ethylene production.103 Fracassini et al. (2002)104 showed the role of a polyamine in corolla senescence of tobacco. Results show that bis-derivatives decreased with the progression of senescence, while mono-derivatives increased during early senescence. These derivatives were present in different amounts in the proximal and distal parts of the corolla. In excised flowers, exogenous spermine delayed senescence and PCD, and caused an increase in polyamine levels.
van Doorn (2004)105 reviewed evidence for sugar starvation to be a cause of petal senescence. Changes in ultrastructure, metabolism and gene expression in senescent petals are remarkably similar to those in sugar-starving organs. The delay in protein degradation and delayed expression of a number of genes was reported after sugar feeding.106 Thus, low sugar levels may elevate ethylene production and trigger petal cell death in flowers. In recent study, Hoeberichts et al. (2007)107 reported that soluble sugars, like sucrose, in the carnation petal act as a repressor of senescence at the transcriptional level. It was observed that sucrose acts more efficiently than the silver thiosulphate (STS an ethylene receptor inhibitor) in ethylene-signaling inhibition. For example the senescence-associated increase in Dc-EIL3 expression was delayed by STS and prevented by sucrose treatment. Sucrose starvation, therefore, might negatively affect ethylene signaling subsequent to SAGs expression.
Senescence-Associated Genes (SAGs) in Flower
Several genes associated with floral senescence have been isolated from a number of flowers using different tools In addition to genes involved in ethylene biosynthesis, there are also reports of expression of some other genes such as a glutathione S-transferase.108 The GST1 from carnation was enhanced by ethylene and contained a 22 bp ethylene response enhancer element that was bound by a 32 kDa protein, CEBP-1.109 The differential display has been used in orchid flowers to isolate three MADS box genes of the AP1/AGL9 subfamily.110 The ubiquitin pathway is also reported to be involved in the degradation of petal proteins during floral senescence in daylily,111 and a proteasome inhibitor, Z-leu-leu-Nva-H, was shown to delay senescence in Iris tepals.112 Xu et al. (2006)113 showed that a tonoplast-localized cytochrome P450 expressed at a higher level in senescing petals of Petunia than in vegetative tissue. Upregulation occurs in response to compatible pollination, ethylene treatment, or jasmonate treatment. Recently Li et al. (2007)114 reported a MAP kinase p56 involved in self-incompability-induced early PCD signalling cascade in incompatible pollen. The p56 activation is necessary for progression of PCD in pollen and use of specific inhibitor of MAPK, U0126, inhibits pollen tube growth.
In Arabidopsis delayed abscission mutants also showed delayed flower senescence. For example delayed floral organ abscission mutants (dab-1, dab-2, dab-3)115 and inflorescence deficient in abscission (ida), in which floral organs remain attached to the plant body after the shedding of mature seeds, showed delayed flower senescence.116 MADS-box genes, like AGL15 and AGL18 (AGAMOUS-like 15 and 18) have also been shown to play an important role in flower senescence. Plants that constitutively overexpress AGL15 exhibit delayed flowering, and delayed floral organ abscission and senescence.117 Another MADS box gene AGL18 over-expression showed prolonged longevity and retention of perianth organs. Thus AGL15 and AGL18 have the capacity to delay floral organ senescence as well as flowering time when expressed at elevated levels.118
Using substractive hybridization 54 genes were isolated from Daffodil, including genes encoding a few regulatory proteins and several cysteine and serine proteases.50 van Doorn et al. (2003)10 through microarray identified a number of genes that were highly expressed during senescence. These included a number of genes with unknown function and sequences encoding a Grap2 and cyclin-D interacting protein, a MADS-domain transcription factor, a casein kinase, and a nucleotide-gated ion channel interacting protein might be important elements in the regulation of senescence. Breeze et al. (2004)119 identified 109 genes associated with flower senescence in Alstroemeria, out of which 93 were upregulated and 16 were downregulated. The upregulated genes encoded e.g., a zinc finger protein, a Xa21 receptor-type protein kinase, and an aspartic proteinase. Among the downregulated genes were sequences encoding a gibberellins-induced protein and a cytochrome P450.
Yamada et al. (2006)120 using differential screening reported several SAGs in morning glory flower senescence. One of them was protein kinase with upregulated expression which may be involved in signalling cascade during senescence as leucine-rich repeat transmembrane protein kinase has been reported to become upregulated, rather specifically, during leaf senescence in Arabidopsis. Another Arabidopsis homologue ataxin2 was also reported during flower senescence. Ataxin2 caused premature cell death in yeast due to defects in actin filament formation.121 This actin depolymerisation is also reported in cell death in pollen tubes.122
Xu et al. (2007)123 reported that the opening and senescence of M. jalapa flowers appears to be under photoperiodic control and using differential screening reported expression of several light-responsive and circadian clock-related transcription factors. The transcription factors significantly upregulated during senescence were homologues of bZIP proteins. A remarkable abundance of transcripts of a gene encoding a RING zinc finger ankyrin protein increased 40,000-fold as the flowers senescent. It might be possible that RING zinc finger protein, that shows such dramatic upregulation during senescence, may play a key role in the control of the process and further detailed study in flower senescence is needed. Hoeberichts et al. (2007)107 using microarray analysis of senesced carnation flowers reported differential expression of several genes especially upregulation of numerous ethylene response genes. A total of 268 genes were analyzed and they mainly grouped in genes associated with protein degradation, cell wall degradation, ROS, lipid degradation, defence and signal transduction.
In general, thousands of genes are regulated in a tissue at a particular time, and hundreds of them may change expression during PCD/senescence. Several associated changes caused by senescence have been difficult to identify because sets of genes are typically involved. Instead of targeting individual genes, it has been necessary to identify groups of genes that coordinate and regulate senescence process synergistically. The large numbers of genes were identified in last few years using several differential tools and showed up and downregulation pattern during the flower senescence. In fact, a number of SAGs identified through differential analysis have yet to be characterized.124 Another most important aspect is that a combination of physiological, biochemical, genetic and molecular assets of SAG products will be required to fully elucidate the regulation of flower senescence.
Conclusions and Perspectives
Flower senescence is a quick and developmentally controlled response, therefore it provides an excellent model system to study the molecular aspects of PCD of plant organs. In genaral, the flower senescence is associated with several structural, physiological, biochemical and molecular changes in the flower senencent organs. These changes include loss of the membrane permiability, leakage of ions, upregulation of oxidative enzymes, generation of ROS, degredation of proteins, lipids, carbohydrates and nucleic acids, imbalance of plant hormones, polyamines, sugars and calcium and finally the up and downregulation of several genes associated with the flower senescence. Several external factors such as light, injury, pollination, temperature and dehydartion also affect the petal senescence. Many components of signal transduction pathways including G-proteins, inositol phosphate, calcium and kinases and phosphatases are also known to play important roles in petal senescence. Petal senescence is usually associated with an increase in the expression of ethylene-regulated genes. In ethylene-regulated PCD mechanism the primary signal initiation and cascade is still not known and it needs to be elucidated. In ethylene-insensitive flowers, though the ABA plays a regulatory role but the exact mechanism is not well known. Several key regulatory genes are known to be involved in the degradation of lipids, proteins, nucleic acids and cell wall components. The roles of catalytic genes were reported in different species with spatial regulation, so it becomes important to remember that different species may have different signal transduction system. A large number of SAGs are identified through different molecular tools but these have not been characterized. The characterization of these up or downregulated genes should also provide useful information. The identification and analysis of promoters of several floral SAGs will be useful for the understanding the regulatory mechanism during flower senescence. Using transgenics/mutants of SAGs the analysis of physiological, biochemical and molecular behaviour will be helpful to elucidate the regulation of flower senescence. There is also a need to study the interacting partners of SAGs encoded proteins, which will help to elucidate the signalling cascade for better understanding of flower senescence mechanism. Also there is a challenge to understand how various external or internal stimuli and developmental factors control the flower senescence.
Acknowledgements
We thank anonymous referee and Dr. Renu Tuteja for critical suggestions on the manuscript. This work was partially supported by the grants from the Department of Biotechnology, Department of Science and Technology and Defence Research and Development Organization, Government of India.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4991
References
- 1.van Doorn WG, Woltering EJ. Senescence and programmed cell death: Substance or semantics? J Exp Bot. 2004;55:2147–2153. doi: 10.1093/jxb/erh264. [DOI] [PubMed] [Google Scholar]
- 2.Rogers Hilary J. Programmed cell death in floral organs: How and why do flowers die? Ann Bot. 2006;97:309–315. doi: 10.1093/aob/mcj051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashman TL, Schoen DJ. How long should flowers live? Nature. 1994;371:788–791. [Google Scholar]
- 4.O'Neill SD. Pollination regulation of flower development. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:547–574. doi: 10.1146/annurev.arplant.48.1.547. [DOI] [PubMed] [Google Scholar]
- 5.ten Have A, Woltering EJ. Ethylene biosynthetic genes are differentially expressed during carnation (Dianthus caryophyllus L.) flower senescence. Plant Mol Biol. 1997;34:89–97. doi: 10.1023/a:1005894703444. [DOI] [PubMed] [Google Scholar]
- 6.Woodson WR, Lawton KA. Ethylene-induced gene expression in carnation petals: Relationship to autocatalytic ethylene production and senescence. Plant Physiol. 1988;87:498–503. doi: 10.1104/pp.87.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleecker AB, Patterson SE. Last exit: Senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell. 1997;9:1169–1179. doi: 10.1105/tpc.9.7.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woltering EJ, van Doorn WG. Role of ethylene and senescence of petals: Morphological and taxonomical relationships. J Exp Bot. 1988;39:1605–1616. [Google Scholar]
- 9.Breeze E, Wagstaff C, Harrison E, Bramke I, Rogers H, Stead A. Gene expression patterns to define stages of post-harvest senescence in Alstroemeria petals. Plant Biotechnol J. 2004;2:155–168. doi: 10.1111/j.1467-7652.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 10.van Doorn WG, Balk PA, van Houwelingen AM, Hoeberichts FA, Hall RD, Vorst O. Gene expression during anthesis and senescence in Iris flowers. Plant Mol Biol. 2003;53:845–863. doi: 10.1023/B:PLAN.0000023670.61059.1d. [DOI] [PubMed] [Google Scholar]
- 11.Thomas H, Ougham HJ, Wagstaff C, Stead AD. Defining senescence and death. J Exp Bot. 2003;54:1127–1132. doi: 10.1093/jxb/erg133. [DOI] [PubMed] [Google Scholar]
- 12.Langston BJ, Bai S, Jones ML. Increases in DNA fragmentation and induction of a senescence-specific nuclease are delayed during corolla senescence in ethylene-insensitive (etr1-1) transgenic Petunias. J Exp Bot. 2005;56:15–23. doi: 10.1093/jxb/eri002. [DOI] [PubMed] [Google Scholar]
- 13.Rieu I, Wolters-Arts M, Derksen J, Mariani C. Ethylene regulates the timing of anther dehiscence in tobacco. Planta. 2003;217:131–137. doi: 10.1007/s00425-003-0976-9. [DOI] [PubMed] [Google Scholar]
- 14.Wagstaff C, Malcolm P, Rafiq A, Leverentz M, Griffiths G, Thomas B. Programmed cell death (PCD) processes begin extremely early in Alstroemeria petal senescence. New Phytologist. 2003;160:49–59. doi: 10.1046/j.1469-8137.2003.00853.x. [DOI] [PubMed] [Google Scholar]
- 15.Christensen CA, Gorsich SW, Brown RH, Jones LG, Brown J, Shaw JM. Mitochondrial GFA2 is required for synergid cell death in Arabidopsis. The Plant Cell. 2002;14:2215–2232. doi: 10.1105/tpc.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Doorn WG, Woltering EJ. Many ways to exit? Cell death categories in plants. Trends Plant Sci. 2005;10:117–122. doi: 10.1016/j.tplants.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Papini A, Mosti S, Brighigna L. Programmed-cell death events during tapetum development of angiosperms. Protoplasma. 1999;207:213–221. [Google Scholar]
- 18.Wang M, Hoekstra S, Van Bergen S, Lamers GEM, Oppedijk BJ, Van der Heijden MW. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol Biol. 1999;39:489–501. doi: 10.1023/a:1006198431596. [DOI] [PubMed] [Google Scholar]
- 19.Balk J, Leaver CJ. The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. The Plant Cell. 2001;13:1803–1818. doi: 10.1105/TPC.010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas S, Franklin-Tong VE. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature. 2004;429:305–309. doi: 10.1038/nature02540. [DOI] [PubMed] [Google Scholar]
- 21.Thomas SG, Huang S, Li S, Staiger CJ, Franklin-Tong VE. Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. J Cell Biol. 2006;174:221–229. doi: 10.1083/jcb.200604011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa NK, Nishimura M. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 2001;42:894–899. doi: 10.1093/pcp/pce144. [DOI] [PubMed] [Google Scholar]
- 23.Jones ML, Chaffin GS, Eason JR, Clark DG. Ethylene-sensitivity regulates proteolytic activity and cysteine protease gene expression in Petunia corollas. J Exp Bot. 2005;56:2733–2744. doi: 10.1093/jxb/eri266. [DOI] [PubMed] [Google Scholar]
- 24.Valpuesta V, Lange NE, Guerrero C, Reid MS. Upregulation of a cysteine protease accompanies the ethylene-insensitive senescence of daylily (Hemerocallis) flowers. Plant Mol Biol. 1995;28:575–582. doi: 10.1007/BF00020403. [DOI] [PubMed] [Google Scholar]
- 25.Cercos M, Santamaria S, Carbonell J. Cloning and characterization of TPE4A, a thiol-protease gene induced during ovary senescence and seed germination in pea. Plant Physiol. 1999;119:1341–1348. doi: 10.1104/pp.119.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page T, Griffiths G, Buchanan-Wollaston V. Molecular and biochemical characterization of postharvest senescence in broccoli. Plant Physiol. 2001;125:718–727. doi: 10.1104/pp.125.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith MT, Saks Y, van Staden J. Ultrastructural changes in the petals of senescing flowers of Dianthus caryophyllus L. Ann Bot. 1992;69:277–285. [Google Scholar]
- 28.Stead AD, van Doorn . Strategies of flower senescence—A review. In: Scott RJ, Stead AD, editors. Molecular and Cellular Aspects of Plant Reproduction. Cambridge, UK: Cambridge University Press; 1994. pp. 215–238. [Google Scholar]
- 29.Thompson JE, Froese CD, Hong Y, Hudak KA, Smith MD. Membrane deterioration during senescence. Can J Bot. 1997;75:867–879. [Google Scholar]
- 30.Phillips HL, Jr, Kende H. Structural changes in flowers of Ipomea tricolor during flower opening and closing. Protoplasma. 1980;102:199–215. [Google Scholar]
- 31.Paliyath G, Droillard MJ. The mechanisms of membrane deterioration and disassembly during senescence. Plant Physiol Biochem. 1992;30:789–812. [Google Scholar]
- 32.Panavas T, Rubinstein B. Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Sci. 1998;133:125–138. [Google Scholar]
- 33.Sylvestre I, Droillard MJ, Bureau JM, Paulin A. Effects of the ethylene rise on the peroxidation of membrane lipids during the senescence of cut carnations. Plant Physiol Biochem. 1989;27:407–413. [Google Scholar]
- 34.Hong Yuwen, Wang Tzann-Wei, Hudak Katalin A, Schade Frank, Froese Carol D, Thompson John E. An ethylene-induced cDNA encoding a lipase expressed at the onset of senescence. PNAS. 2000;97:8717–8722. doi: 10.1073/pnas.140213697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leverentz Michael K, Wagstaff Carol, Rogers Hilary J, Stead Anthony D, Chanasut Usawadee, Silkowski Helena, Thomas Brian, Weichert Heiko, Feussner Ivo, Griffiths Gareth. Characterization of a novel lipoxygenase-independent senescence mechanism in Alstroemeria peruviana floral tissue. Plant Physiol. 2002;130:273–283. doi: 10.1104/pp.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuchi-Mizutani M, Ishiguro K, Nakayama T, Utsunomiya Y, Tanaka Y, Kusumi T, Ueda T. Molecular and functional characterization of a rose lipoxygenase cDNA related to flower senescence. Plant Sci. 2000;160:129–137. doi: 10.1016/s0168-9452(00)00373-3. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JE, Froese CD, Madey E, Smith MD, Hong YW. Lipid metabolism during plant senescence. Prog Lipid Res. 1998;37:119–141. doi: 10.1016/s0163-7827(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 38.Porat R, Halevy AH, Serek M, Borochov A. An increase in ethylene sensitivity following pollination is the initial event triggering an increase in ethylene production and enhanced senescence of Phalaenopsis orchid flowers. Physiologia Plantarum. 1995;93(4):778–784. [Google Scholar]
- 39.Rubinstein B. Regulation of cell death in flower petals. Plant Mol Biol. 2000;44:303–318. doi: 10.1023/a:1026540524990. [DOI] [PubMed] [Google Scholar]
- 40.Peary JS, Prince TA. Floral lipoxygenase: Activity during senescence and inhibition by phenidone. J Am Soc Hortic Sci. 1990;115:455–457. [Google Scholar]
- 41.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Clarendon Press; 1989. pp. 450–499. [Google Scholar]
- 42.Bartoli CG, Simontacchi M, Guiamet J, Montaldi E, Puntarulo S. Antioxidant enzymes and lipid peroxidation during aging of Chrysanthemum morifolium RAM petals. Plant Sci. 1995;104:161–168. [Google Scholar]
- 43.del Rio LA, Palma JM, Sandalio LM, Corpas FJ, Pastori GM, Bueno P, Lopez-Huertas E. Peroxisomes as a source of superoxide and hydrogen peroxide in stressed plants. Biochem Soc Trans. 1996;24:434–438. doi: 10.1042/bst0240434. [DOI] [PubMed] [Google Scholar]
- 44.Bartoli CG, Simontacchi M, Montaldi E, Puntarulo S. Oxidants and antioxidants during ageing of chrysanthemum petals. Plant Sci. 1997;129:157–165. [Google Scholar]
- 45.Beja-Tal S, Borochov A. Age-related changes in biochemical and physical properties of carnation petal plasma membranes. J Plant Physiol. 1994;143:195–199. [Google Scholar]
- 46.Beers EP, Woffenden BJ, Zhao C. Plant proteolytic enzymes: Possible roles during programmed cell death. Plant Mol Biol. 2000;44:399–415. doi: 10.1023/a:1026556928624. [DOI] [PubMed] [Google Scholar]
- 47.Stephenson P, Rubinstein B. Characterization of proteolytic activity during senescence in daylilies. Physiologia Plantarum. 1998;104:463–473. [Google Scholar]
- 48.Wagstaff C, Leverentz MK, Griffiths G, Thomas B, Chanasut U, Stead AD, Rogers HJ. Cysteine protease gene expression and proteolytic activity during senescence of Alstroemeria petals. J Exp Bot. 2002;53:233–240. doi: 10.1093/jexbot/53.367.233. [DOI] [PubMed] [Google Scholar]
- 49.Eason JR, Ryan DJ, Pinkney TT, O'Donoghue EM. Programmed cell death during flower senescence: Isolation and characterization of cysteine proteases from Sandersonia aurantiaca. Funct Plant Biol. 2002;29:1055–1064. doi: 10.1071/PP01174. [DOI] [PubMed] [Google Scholar]
- 50.Hunter DA, Steele BC, Reid MS. Identification of genes associated with perianth senescence in Daffodil (Narcissus pseudonarcissus L. “Dutch Master”) Plant Sci. 2002;163:13–21. [Google Scholar]
- 51.Guerrero C, de la Calle M, Reid MS, Valpuesta V. Analysis of the expression of two thiolprotease genes from daylily (Hemerocallis spp.) during flower senescence. Plant Mol Biol. 1998;36:656–671. doi: 10.1023/a:1005952005739. [DOI] [PubMed] [Google Scholar]
- 52.Azeez A, Sane AP, Bhatnagar D, Nath P. Enhanced expression of serine proteases during floral senescence in Gladiolus. Phytochemistry. 2007;68:1352–1357. doi: 10.1016/j.phytochem.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 53.Jones ML, Larsen PB, Woodson WR. Ethylene-regulated expression of a carnation cysteine proteinase during flower petal senescence. Plant Mol Biol. 1995;28:505–512. doi: 10.1007/BF00020397. [DOI] [PubMed] [Google Scholar]
- 54.Sugawara H, Shibuya K, Yoshioka T, Hashiba T, Satoh S. Is a cysteine proteinase inhibitor involved in the regulation of petal wilting in senescing carnation (Dianthus caryophyllus L.) flowers? J Exp Bot. 2002;53:407–413. doi: 10.1093/jexbot/53.368.407. [DOI] [PubMed] [Google Scholar]
- 55.Panavas T, Pikula A, Reid PD, Rubinstein B, Walker EL. Identification of senescence-associated genes from daylily petals. Plant Mol Biol. 1999;40:237–248. doi: 10.1023/a:1006146230602. [DOI] [PubMed] [Google Scholar]
- 56.Xu Y, Hanson MR. Programmed cell death during pollination induced petal senescence in Petunia. Plant Physiol. 2000;122:1323–1333. doi: 10.1104/pp.122.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada T, Takatsu Y, Kasumi M, Ichimura K, van Doorn WG. Nuclear fragmentation and DNA degradation during programmed cell death in petals of morning glory (Ipomoea nil) Planta. 2006;224:1279–1290. doi: 10.1007/s00425-006-0307-z. [DOI] [PubMed] [Google Scholar]
- 58.Taylor CB, Bariola PA, DelCardayre SB, Raines RT, Green PJ. A senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proc Natl Acad Sci USA. 1993;90:5118–5122. doi: 10.1073/pnas.90.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lers A, Khalchitski A, Lomaniec E, Burd S, Green PJ. Senescence-induced RNases in tomato. Plant Mol Biol. 1998;36:439–449. doi: 10.1023/a:1005993024161. [DOI] [PubMed] [Google Scholar]
- 60.Yamada Tetsuya, Ichimura Kazuo, van Doorn Wouter G. DNA degradation and nuclear degeneration during programmed cell death in petals of Antirrhinum, Argyranthemum, and Petunia. J Exp Bot. 2006;57:3543–3552. doi: 10.1093/jxb/erl100. [DOI] [PubMed] [Google Scholar]
- 61.Taylor JE, Whitelaw CA. Signals in abscission. New Phytol. 2001;151:323–339. 138. [Google Scholar]
- 62.Stead AD. Pollination-induced flower senescence: A review. Plant Growth Regul. 1992;11:13–20. [Google Scholar]
- 63.Woltering EJ, ten Have A, Larsen PB, Woodson WR. Ethylene biosynthetic genes and interorgan signalling during flower senescence. In: Scott RJ, Stead AD, editors. Molecular and Cellular Aspects of Plant Reproduction. Cambridge, UK: Cambridge University Press; 1994. pp. 285–307. [Google Scholar]
- 64.van Doorn WG, Stead AD. Abscission of flowers and floral parts. J Exp Bot. 1997;48:821–837. [Google Scholar]
- 65.Larsen PB, Ashworth EN, Jones ML, Woodson WR. Pollination-induced ethylene in carnation. Plant Physiol. 1995;108:1405–1412. doi: 10.1104/pp.108.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh A, Evenson KB, Kao TH. Ethylene synthesis and floral senescence following compatible and incompatible pollinations in Petunia inflata. Plant Physiol. 1992;99:38–45. doi: 10.1104/pp.99.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bui AQ, O'Neill SD. Three 1-aminocyclopropane-1-carboxylate-synthase genes regulated by primary and secondary pollination signals in orchid flowers. Plant Physiol. 1998;116:419–428. doi: 10.1104/pp.116.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woltering EJ, de Vrije T, Harren F, Hoekstra FA. Pollination and stigma wounding: Same response, different signal? J Exp Bot. 1997;48:1027–1033. [Google Scholar]
- 69.Whitehead CS. Ethylene sensitivity and flower senescence. In: Scott RJ, Stead AD, editors. Molecular and Cellular Aspects of Plant Reproduction. Cambridge, UK: Cambridge University Press; 1994. pp. 269–284. [Google Scholar]
- 70.Whitehead CS, Vasiljevic D. Role of short-chain saturated fatty acids in the control of ethylene sensitivity in senescing carnation flowers. Physiol Plant. 1993;88:243–250. [Google Scholar]
- 71.Hunter Donald Alexander, Ferrante Antonio, Vernieri Paolo, Stuart Michael. Role of abscisic acid in perianth senescence of Daffodil (Narcissus pseudonarcissus “Dutch Master“) Physiologia Plantarum. 2004;121:313–321. doi: 10.1111/j.0031-9317.2004.0311.x. [DOI] [PubMed] [Google Scholar]
- 72.Beja-Tal S, Borochov A, Gindin E, Mayak S. Transient water stress in cut carnation flowers: Effects of cycloheximide. Scient Hort. 1995;64:167–175. [Google Scholar]
- 73.Garello G, Menard C, Dansereau B, LePage-Degivry MT. The influence of light quality on rose flower senescence: Involvement of abscisic acid. Plant Growth Regul. 1995;16:135–139. [Google Scholar]
- 74.Woltering EJ, de Vrije T, Harren F, Hoekstra FA. Pollination and stigma wounding: Same response, different signal? J Exp Bot. 1997;48:1027–1033. [Google Scholar]
- 75.Vierling E. The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:579–620. [Google Scholar]
- 76.Verlinden S, Woodson WR. The physiological and molecular responses of carnation flowers to high temperature. Postharvest Biol Techn. 1998;4:185–192. [Google Scholar]
- 77.Tang X, Gomes AMTR, Bhatia A, Woodson WR. Pistil-specific and ethylene-regulated expression of 1-aminocyclopropane-1-carboxylate oxidase genes in Petunia flowers. Plant Cell. 1994;6:1227–1239. doi: 10.1105/tpc.6.9.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang X, Woodson WR. Temporal and spatial expression of 1-aminocyclopropane-1-carboxylate oxidase mRNA following pollination of immature and mature Petunia flowers. Plant Physiol. 1996;112:503–511. doi: 10.1104/pp.112.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nadeau JA, Zhang XS, Nair H, O'Neill SD. Temporal and spatial regulation of 1-aminocyclopropane-1-carboxylate oxidase in the pollination-induced senescence of orchid flowers. Plant Physiol. 1993;103:31–39. doi: 10.1104/pp.103.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shibuya K, Nagata M, Tanikawa N, Yoshioka T, Hashiba T, Satoh S. Comparison of mRNA levels of three ethylene receptors in senescing flowers of carnation (Dianthus caryophyllus L.) J Exp Bot. 2002;53:399–406. doi: 10.1093/jexbot/53.368.399. [DOI] [PubMed] [Google Scholar]
- 81.Müller R, Lind-Iversen S, Stummann BM, Serek M. Expression of genes for ethylene biosynthetic enzymes and an ethylene receptor in senescing flowers of miniature potted roses. J Hortic Sci Biotechnol. 2000;75:12–18. [Google Scholar]
- 82.Kuroda S, Hirose Y, Shiraishi M, Davies E, Abe S. Co-expression of an ethylene receptor gene, ERS1, and ethylene signaling regulator gene, CTR1, in Delphinium during abscission of florets. Plant Physiol Biochem. 2004;42:745–751. doi: 10.1016/j.plaphy.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Müller R, Owen CA, Xue ZT, Welander M, Stummann BM. Characterization of two CTR-like protein kinases in Rosa hybrida and their expression during flower senescence and in response to ethylene. J Exp Bot. 2002;53:1223–1225. doi: 10.1093/jexbot/53.371.1223. [DOI] [PubMed] [Google Scholar]
- 84.Waki K, Shibuya K, Yoshioka T, Hashiba T, Satoh S. Cloning of a cDNA encoding EIN3-like protein (DC-EIL1) and decrease in its mRNA level during senescence in carnation flower tissues. J Exp Bot. 2001;355:377–379. [PubMed] [Google Scholar]
- 85.Müller R, Stummann BM, Serek M. Characterization of an ethylene receptor family with differential expression in rose (Rosa hybrida L.) flowers. Plant Cell Rep. 2000;19:1232–1239. doi: 10.1007/s002990000251. [DOI] [PubMed] [Google Scholar]
- 86.Savin KW, Baudinette SC, Graham MW, Michael ZM, Nugent GD, Lu CY, Chandler SF, Cornish EC. Antisense ACC oxidase RNA delays carnation petal senescence. Hort Science. 1995;30:970–972. [Google Scholar]
- 87.Bovy AG, Angenent GC, Dons HJM, van Altvorst AC. Heterologous expression of the Arabidopsis etr1-1 allele inhibits the senescence of carnation flowers. Mol Breed. 1999;5:301–308. [Google Scholar]
- 88.Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- 89.Borochov A, Woodson WR. Physiology and biochemistry of flower petal senescence. Hort Rev. 1989;11:15–43. [Google Scholar]
- 90.Panavas T, Rubinstein B. Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Sci. 1998;133:125–138. [Google Scholar]
- 91.Ronen M, Mayak S. Interrelationship between abscisic acid and ethylene in the control of senescence processes in carnation flowers. J Exp Bot. 1981;32:759–765. [Google Scholar]
- 92.Hunter DA, Ferrante A, Vernieri P, Reid MS. Role of abscisic acid in perianth senescence of Daffodil (Narcissus pseudonarcissus “Dutch Master”) Physiol Plant. 2004;121:313–321. doi: 10.1111/j.0031-9317.2004.0311.x. [DOI] [PubMed] [Google Scholar]
- 93.Lara MEB, Garcia MCG, Fatima T, Ehness R, Lee TK, Proels R. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell. 2004;16:1276–1287. doi: 10.1105/tpc.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mayak S, Halevy AH. Interrelationships of ethylene and abscisic acid in the control of rose petal senescence. Plant Physiol. 1972;50:341–346. doi: 10.1104/pp.50.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Staden J, Dimalla GG. The effect of silver thiosulfate preservative on the physiology of cut carnations: II. Influence of endogenous cytokinins. Z Pflanzenphysiol. 1980;99:19–26. [Google Scholar]
- 96.Chang H, Jones M, Banowetz GM, Clark DG. Overproduction of cytokinins in Petunia flowers transformed with PSAG12-IPT delays corolla senescence and decreases sensitivity to ethylene. Plant Physiol. 2003;132:2174–2183. doi: 10.1104/pp.103.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saks Y, Van Staden J, Smith MT. Effect of gibberellic acid on carnation flower senescence evidence that the delay of carnation flower senescence by gibberellic acid depends on the stage of flower development. Plant Growth Regul. 1992;11:45–51. [Google Scholar]
- 98.Setyadjit, Irvin DE, Joyce DC, Simons DH. Vase treatments containing gibberellic acid do not increase longevity of cut Sylvia' inflorescences. Australian Journal of Experimental Agriculture. 2006;46:1535–1539. [Google Scholar]
- 99.Borochov A, Cho MH, Boss WF. Plasma membrane lipid metabolism of Petunia petals during senescence. Physiol Plant. 1994;90:279–284. [Google Scholar]
- 100.Borochov A, Spiegelstein H, Philosoph-Hadas S. Ethylene and flower petal senescence: Interrelationship with membrane lipid catabolism. Physiol Plant. 1997;100:606–612. [Google Scholar]
- 101.Huang FY, Philosoph-Hadas S, Meir S, Callahan DA, Sabato R, Zelcer A, Hepler PK. Increases in cytosolic Ca2C in parsley mesophyll cells correlated with leaf senescence. Plant Physiol. 1997;115:51–60. doi: 10.1104/pp.115.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Porat R, Borochov A, Halevy AH. Pollinationinduced senescence in Phalaenopsis petals: Relationship of ethylene sensitivity to activity of GTP-binding proteins and protein phosphorylation. Physiol Plant. 1994;90:679–684. [Google Scholar]
- 103.Lee M, Lee SH, Park KY. Effects of spermine on ethylene biosynthesis in cut carnation (Dianthus caryophyllus L.) flowers during senescence. J Plant Physiol. 1997;151:68–73. [Google Scholar]
- 104.Serafini-Fracassini D, Del Duca S, Monti F, Poli F, Sacchetti G, Bregoli AM, Biondi S, Della Mea M. Transglutaminase activity during senescence and programmed cell death in the corolla of tobacco (Nicotiana tabacum) flowers. Cell Death Differ. 2002;9:309–321. doi: 10.1038/sj.cdd.4400954. [DOI] [PubMed] [Google Scholar]
- 105.van Doorn WG. Is petal senescence due to sugar starvation? Plant Physiol. 2004;134:35–42. doi: 10.1104/pp.103.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eason JR, de Vre LA, Somerfield SD, Heyes JA. Physiological changes associated with Sandersonia aurantiaca flower senescence in response to sugar. Postharvest Biol Technol. 1997;12:43–50. [Google Scholar]
- 107.Hoeberichts FA, van Doorn WG, Vorst O, Hall RD, van Wordragen MF. Sucrose prevents upregulation of senescence-associated genes in carnation petals. J Exp Bot. 2007 doi: 10.1093/jxb/erm076. In press. [DOI] [PubMed] [Google Scholar]
- 108.Meyer RC, Goldsbrough PB, Woodson WR. An ethylene-responsive flower senescence-related gene from carnation encodes a protein homologous to glutathione S-transferases. Plant Mol Biol. 1991;17:277–281. doi: 10.1007/BF00039505. [DOI] [PubMed] [Google Scholar]
- 109.Maxson JM, Woodson WR. Cloning of a DNA-binding protein that interacts with the ethylene-responsive enhancer element of the carnation GST1 gene. Plant Mol Biol. 1996;31:751–759. doi: 10.1007/BF00019463. [DOI] [PubMed] [Google Scholar]
- 110.Yu H, Goh CJ. Identification and characterization of three orchid MADS-box genes of the AP1/AGL9 subfamily during floral transition. Plant Physiol. 2000;123:1325–1336. doi: 10.1104/pp.123.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Courtney SE, Rider CC, Stead AD. Changes in protein ubiquitination and the expression of ubiquitin-encoding transcripts in daylily petals during floral development and senescence. Physiol Plant. 1994;91:196–204. [Google Scholar]
- 112.Pak C, van Doorn WG. Delay of Iris flower senescence by protease inhibitors. New Phytol. 2005;165:473–480. doi: 10.1111/j.1469-8137.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- 113.Yan Xu, Ishida Hiroyuki, Reisen Daniel, Hanson Maureen R. Upregulation of a tonoplast-localized cytochrome P450 during petal senescence in Petunia inflate. BMC Plant Biology. 2006;6:8. doi: 10.1186/1471-2229-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shutian Li, Šamaj Jozef, Franklin-Tong Vernonica E. A MAPK signals to programmed cell death induced by self-incompatibility in Papaver pollen. Plant Physiol. 2007;145:236–245. doi: 10.1104/pp.107.101741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patterson SE, Bleecker AB. Ethylene-dependent and -independent processes associated with floral organ abscission in Arabidopsis. Plant Physiol. 2004;134:194–203. doi: 10.1104/pp.103.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Butenko MA, Patterson SE, Grini PE, Stenvik GE, Amundsen SS, Mandal A, Aalen RB. Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell. 2003;15:2296–2307. doi: 10.1105/tpc.014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fernandez DE, Heck GR, Perry SE, Patterson SE, Bleecker AB, Fang SC. The embryo MADS domain factor AGL15 acts postembryonically: Inhibition of perianth senescence and abscission via constitutive expression. Plant Cell. 2000;12:183–198. doi: 10.1105/tpc.12.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adamczyk, Lehti-Shiu Melissa D, Fernandez Donna E. The MADS domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. The Plant Journal. 2007;50:1007–1019. doi: 10.1111/j.1365-313X.2007.03105.x. [DOI] [PubMed] [Google Scholar]
- 119.Breeze E, Wagstaff C, Harrison E, Bramke I, Rogers H, Stead A. Gene expression patterns to define stages of post-harvest senescence in Alstroemeria petals. Plant Biotechnol J. 2004;2:155–168. doi: 10.1111/j.1467-7652.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 120.Tetsuya Yamada, Ichimura Kazuo, Kanekatsu Motoki, van Doorn outer G. Gene expression in opening and senescing petals of morning glory (Ipomoea nil) flowers. Plant Cell Reports. 2007;26:823–835. doi: 10.1007/s00299-006-0285-4. [DOI] [PubMed] [Google Scholar]
- 121.Satterfield TF, Jackson SM, Pallanck LJ. A Drosophila homolog of the polyglutamine disease gene SCA2 is a dosage-sensitive regulator of actin filament formation. Genetics. 2002;162:1687–1702. doi: 10.1093/genetics/162.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomas SG, Huang S, Li S, Staiger CJ, Franklin-Tong VE. Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. J Cell Biol. 2006;174:221–229. doi: 10.1083/jcb.200604011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xinjia Xu, Gookin Tim, Jiang Cai-Zhong, Reid Michael. Genes associated with opening and senescence of Mirabilis jalapa flowers. J Exp Bot. 2007;58:2193–2201. doi: 10.1093/jxb/erm058. [DOI] [PubMed] [Google Scholar]
- 124.Marianne Hopkins, Taylor Catherine, Liu Zhongda, Ma Fengshan, McNamara Linda, Wang Tzann-Wei, Thompson John E. Regulation and execution of molecular disassembly and catabolism during. Senescence New Phytol. 2007;175:201–214. doi: 10.1111/j.1469-8137.2007.02118.x. [DOI] [PubMed] [Google Scholar]