Abstract
Special red cells were found on the adaxial surface of tertiary pulvini of Mimosa pudica and experiments performed to determine the origin and function of these cells. Using anatomical (light, scanning electron and transmission electron microscopy) and electrophysiological techniques, we have demonstrated that these red cells are real mechanoreceptor cells. They can generate receptor potential following mechanical stimuli and they are in connection with excitable motor cells (through plasmodesmata). We also provide evidence that these red cells are derived from stomatal subsidiary cells and not guard cells. As histochemical studies show red cells contain tannin, which is important in development of action potentials and movements of plants. These cells could be one of unidentified mechanoreceptors of mimosa.
Key Words: mimosa, mechanoreceptor cells, microscopy, electrophysiology, receptor potential
Introduction
Mimosa is famous for its rapid leaf movements. The leaves close up and droop when touched and reopen within a few minutes. As a result of this spectacular movement, mimosa has been the subject of many studies since the 18th century,1 but there are still many questions in connection with the movement of mimosa, for example, what cells are the mechanoreceptors.2
It is known that movements of mimosa are governed by quick turgor pressure changes through vacuolar systems. Cells of pulvinar motor tissue have two different types of vacuoles, tannin-containing and tannin less vacuoles.3 Several data demonstrate that the large central vacuole, that does not contain tannin, is a contractile vacuole.4 Toriyama and Satô investigated a fine fibrillar structure in the central vacuole of motor cells.5,6 They reported on the cooperation between tannin and the central vacuole. In that, after stimulation, the fine fibrillar structure becomes an aggregated structure.7 This double vacuolar system is a characteristic sign of excitable motor cells.
The contractile mechanism can be involved in the seismonastic reaction of the pulvinar motor cells,8 and describes movements of plants involving a process based not primarily on contractile proteins.9 Most data show that potassium salts caused turgor pressure changes in motor cells.10 Histochemically, detectable potassium salts are found in the motor cells before seismonastic response, but the large crystals of the salts appear in the intercellular spaces after response.11 The power of motor cells, which is transformed into leaf movements, derives from osmotic pressure of potassium and chloride ions.12 Chloride and potassium efflux were measured on the primery pulvinus successfully, with microelectrodes, during seismonastic reaction.13 Hollins and Jaffe, using comparative histochemistry have shown that tannin vacuoles can also store calcium.14 These tannin vacuoles are analogous to sarcoplasmatic reticulum in muscle sarcomers that release Ca2+ into the cytoplasm. In mimosa calcium can cause actomyosin microfilaments to contract, and this contraction can open putative potassium channels.9 As electron micrographs show the osmiophilic tannin vacuolar contents and colloidal contents are sometimes associated (colloidal theory),15 but it is not known whether these interactions are artefacts of fixation or whether they are physiologically significant.
Most of the authors agree that mechanical stimuli are translated to membrane potential changes and at the end this electrical signal is necessary for movements of motor cells.2,11,13,16–19 Some results, however, indicate that the role of organic molecules, i.e., hormones (turgorins) may be the most important factors in this signal transduction.20,21,22
Nowadays, the occurrence of action potentials in plants is a known phenomenon and described frequently.23 In plants (like animals) action potentials can be measured after external stimuli, but action potentials can also be generated spontaneously.24 The first description of an ‘action potential’ was an electric disturbance following stimulation of a leaf of Dionaea muscipulata.25 In case of Mimosa pudica, action potentials were demonstrated in 1962.26 The first review about this topic was published in 1973.16 In that article two different types of membrane potential changes of mimosa were described, action potential (evoked by mechanical stimulation or with cold water application) and variation potential or slow wave potential (after wounding). This variation potential can be a connection with wound hormone of Ricca.27,28 These two types of potential changes of mimosa were measured exactly by the techniques of aphid stylet.17,19
There is a very good understanding about the mechanism of action potential generation in animals, Na+ and K+ ions play a key role in it. Action potential develops and propagates as an impulse in the axon of neurons; with ‘all-or-none’ manner. In plants the most important ions in this process are Ca2+ and Cl−.29 After arrival of the stimulus, free Ca2+ concentration in the cytoplasm increases. This Ca2+ originates from extracellular and intracellular spaces, through voltage dependent ion channels and from vacuoles via secondary transduction pathways. Depolarisation occurs due to Ca2+ activation of Ca2+-dependent anion channels and massive efflux of Cl−. Depolarization leads to opening of K+-channels, and the K+ efflux repolarizes the plasma membrane.30 The characteristics of action potential can be modified by changing of Cl− or K+ concentration.31 Electrical signals may pass through gap junctions (electrical synapses) at speed of 2 to 500 mm/s in animals. In plants, most cells have cell-to-cell conduction through plasmodesmata, and this connection has high solute permeability and electrical conductivity. Plasmodesmata are nearly identical to gap junctions of animal tissues.32 Plasmodesmata can be “synapses of plants,” and from a special point of view auxin can be the mediator/neurotransmitter.33 In plants, ionotropic glutamate receptors were also found, for example in Arabidopsis thaliana.34 These glutamate receptors, similar to the glutamate receptors in the nervous system of animals, can contribute to electrical signal transduction in plants. Experimental data indicate that adding 1 mM glutamate into Arabidopsis seedlings' growth medium causes a rapid increase in cytosolic free Ca2+ and also membrane depolarization.35
Electrical activity in plants (action potentials, variation potential and voltage transients) can be a mechanism for signal propagation and takes place in numerous physiological responses at the molecular and systemic level.36 The role of action potentials is described in case of induction of systemic proteinase inhibitor.32,37–40 Data have been published about the importance of electrical signals in reproductive systems41 and also in the electrical perception and ‘death message’ in Chara cells.42,43
In case of mimosa, the action potential passes in the phloem tubes.17,19,44 When the action potential arrives to the motor cells, the impulse can control motor cell movements through voltage dependent ion channels, and the aquaporins, and blockage of the proton ATPase.18,45 The potential change and its characteristic is one of the most important factors in the control of motor cells. The membrane potential changes are generated in unidentified mechanoreceptor cells as a receptor potential. This electrical stimulus spreads and coordinates the movements of motor cells in mimosa. The mechanoreceptors responsible for the receptor potential in mimosa are unknown. However, stretch activated ion channels in Chara cells have been identified as mechanoreceptors.46
The goal of the present study was to describe mechanosensitive cells on the tertiary pulvinus of Mimosa pudica L. and investigate how they generate a receptor potential that is adequate for motor cell stimulation.
Materials and Methods
Plant material.
Plants of Mimosa pudica L. were grown in the greenhouse at 25°C in soil culture. Leaves for electrophysiological studies were cut off at the petiole between the primary and secondary pulvinus or between the secondary and tertiary pulvinus, and these leaflets were placed on the surface of ¼-strength Hoagland solution in Petri dishes. Ex vivo leaves were left for one or two days floating on the ¼-strength Hoagland solution to stabilize all spontaneous ion fluxes before measurements were made.
Microscopy.
For electron microscopy, leaflets were fixed in 2.5% glutaraldehyde buffered with 0.1 M potassium sodium phosphate, pH 7.2. Tissues were postfixed in 1% OsO4, dehydrated in an ethanol series, and embedded in Spurr or Durcupan epoxi resin. Thin sections (70 nm) were cut by Reichert Ultracut E ultramicrotome. Sections were stained with 5% uranyl acetate and lead citrate47 and examined using a Hitachi 7100 transmission electron microscope at 75 kV.
For scanning electron microscopy, the fixed and dehydrated leaf pieces were transferred to iso-amyl acetate and than dried with a Polaron CPD 7501 critical-point dryer. Samples finally were coated with gold in a Zeiss vacuum evaporator. Observations were made by Hitachi 2360N scanning electron microscope at 18 kV.
For light microscopy, semi-thin sections (1 µm) were cut with Zeiss Hm 360 microtome, then stained with toluidine blue (pH 4.4) and examined using Olympus BH-2 light microscope. To identify tannin, samples were stained with FeSO4 according to Ruzin48 and were studied with Zeiss SMX stereo microscope and Olympus BH-2 light microscope for examination.
Electrophysiology.
Receptor potentials in the red cells were recorded with glass microelectrodes (8–10 MΩ) filled with 3 M KCl and connected through an Ag/AgCl electrode to the amplifier. Recording microelectrodes were fabricated with a horizontal electrode puller Sutter P-97 from 0.75 mm-diameter borosilicate micro-capillaries (Medical System). Measures were performed in a Faraday cage. During the whole experiment the same light intensity was ensured with Tungsram bulb (Tungsraflex R63). One-fourth-strength Hoagland solution was supplied with peristaltic pump (Pharmacia). Recording electrodes were positioned with a Märzhauser micromanipulator under stereomicroscope control (Zeiss SMX). Reference electrode was placed in the bottom of recording chamber in contact with the ¼-strength Hoagland solution surrounding the plant material. After insertion of recording electrode into the cell there was a 20 minute wait before initiating mechanical stimulation. Signals were amplified (Bioamp, Supertech, Hungary) and displayed on a digital oscilloscope (Gould DSO 420, Gould Electronics). Recorded signals were digitized online with an A/D converter (VR-10, Instrutech Corp.) and stored on a VHS tape for further analysis.
Stored signals were analysed with S.P.E.L. advanced Intrasys program (Experimetria) and plotted in Origin 5.0 (Microcal) program.
Results
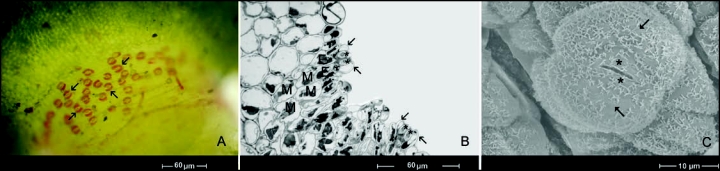
Upon examination of the seismonastic reactions of the sensitive plant, we identified some special, previously undescribed cells on the tertiary pulvinus of Mimosa pudica L. These cells have bright red color without any staining, and at first sight they resemble stomatal guard cells (Fig. 1A). Histochemical studies revealed that these special red cells contain tannin. The tertiary pulvini were fixed in the presence of FeSO4 and the bright red coloration changed into black or deep blue, which is a typical reaction of tannin.
Figure 1.
Red receptor cells (without staining) on tertiary pulvinus of Mimosa pudica resemble stomatal guard cells. Arrows show these bright red cells (A) Receptor cells and receptor complex on tertiary pulvinus of mimosa by light microscope (B) and by scanning electron microscope (C). Arrows point to red receptor cells, stars indicate guard cells (out of work), E, elongated excitable motor cells; M, motor cells.
The presence of red stomatal guard cells on the adaxial part of the tertiary pulvini was very strange. Therefore, we determined to examine the nature and role of these cells. We discovered that the location of these cells completely overlapped the area that is sensible to fine mechanical stimulation. Thus we were interested in their role with respect to the seismonastic reaction. These red cells seemed to be good candidates for the unknown mechanoreceptor cells.
To produce fine, direct mechanical stimuli different types of epidermal cells (trichome, epidermis cells and the specialised red cells) were stimulated with a micromanipulator needle. When trichomes or epidermis cells were touched, there was no reaction, but when the red cells were stimulated, the leaflet closed.
We then explored the anatomical structure of these mechanosensitive cells. Results from light microscopy (Fig. 1B), and scanning electron microscopy (Fig. 1C) studies certified that the red cells do not derive from stomatal guard cells. They appear to derive from stomatal subsidiary cells.
Motor cell movement requires special membrane potential changes, so a real mechanoreceptor cell has to produce a receptor potential, and that receptor potential should propagate to the motor cells. In our electrophysiological investigations changes of membrane potential were measured after mechanical stimulation. Development of receptor potential through the plasma membrane was demonstrated (Fig. 2). As the diagram shows, after the artifact of stimulation, first hyper- than depolarisation and finally repolarisation was detected. The receptor potential measured in agreement with the potential changes measured in motor cells.13 Several of these recordings (more than 15) were made, potential changes varied between 10–12 mV.
Figure 2.
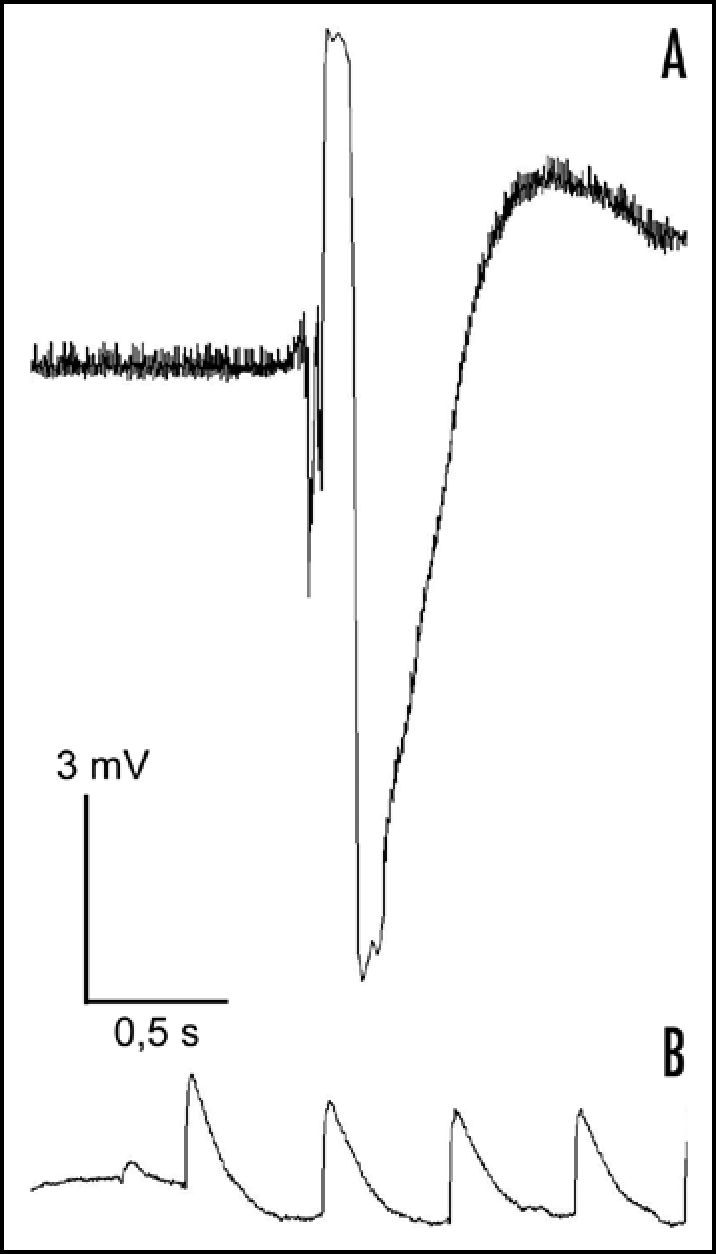
A representative receptor potential on red receptor cells after mechanical stimuli (A) and electrical record on an epidermal cell near to adaxial part of tertiary pulvinus of mimosa. (B) The resting membrane potential of receptor cells was about −90–100 mV and on a simple epidermis cell that was about −30–40 mV.
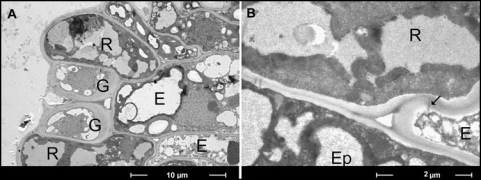
Finally, we investigated whether there were connections between the receptor cells and the motor cells and what the nature of this connection was. The answer was yielded by transmission electron microscopy. The receptor complex consists of two guard cells between two subsidiary cells (Fig. 3A). Under the receptors, motor cell-like elongated cells are situated, with typical double vacuolar system. Moreover, as the electron micrographs show, the receptor cells and excitable cells are connected through plasmodesmata (Fig. 3B). Electrical signals can easily pass down this path.19 These data strongly implicate these red cells as real mechanoreceptor cells.
Figure 3.
Receptor complex on tertiary pulvinus of mimosa by transmission electron microscope (A) and plasmodesmata (arrow) between the red receptor cell and the elongated excitable motor cell (B); R, red receptor cells; E, elongated excitable motor cells; G, guard cells (out of work); Ep, normal epidermis cell.
Discussion
We have found red mechanoreceptive cells on the tertiary pulvini of Mimosa pudica L. The stimulation of these red cells with a micromanipulator needle proved that the stimulus evoked the closure of leaflets.
Histochemical studies show that the red coloration of the receptor cells is due to their tannin content. Tannin vacuoles can function similarly to the sarcoplasmatic reticulum of animal muscle cells. They are huge calcium stores, and after stimulation they are able to release calcium ions.9,14 Similarly, the red cells would be able to produce a receptor potential which is essential for functioning as a mechanoreceptor cell. By means of changes in membrane potential the receptor cells are able to control the pulvinar motor cells. Additionally, as electron micrographs reveal, the receptor and motor cells are in connection through plasmodesmata, which is an electric junction between these two different types of cells.32
Both electron microscopy studies and stimulation with glass pin confirmed that these cells differ from the surrounding epidermal cells. After the stimulation of these cells the tertiary pulvinus closes. One of the most important questions was whether the red receptor cells can produce a receptor potential that can pass down to motor cells. As our electrophysiological measurements demonstrated, after mechanical stimulus a receptor potential is generated in these cells. This receptor potential can pass through plasmodesmata, which are able to control the movements of motor cells.18,45 These results supported our original hypothesis that the red cells on the adaxial part of the tertiary pulvini in Mimosa pudica L. are real receptor cells. They sense the mechanical stimuli and operate the closure mechanism of leaflets.
A question which arises is why stomatal subsidiary cells have transformed to mechanoreceptor cells. Subsidiary cells in the stomatal complex play a role in the control of guard cells taking part in ion channel-mediated opening and closing of the stomatal pore.49 It is well known that electrical changes can be measured on the plasma membrane of subsidiary cells during stomatal movements.50 Potassium and chloride flux between guard cells and subsidiary cells were also observed.51 Stomatal guard cells and pulvinar motor cells in mimosa operate with the same mechanism,52 so it seems logical that stomatal subsidiary cells, that normally coordinate the movements of stomatal guard cells, are modified to control the pulvinar motor cells. However, the question of how they became sensitive for mechanical stimuli remains unanswered. Stretch activated ion channels are likely to play an essential role in mechanoperception. These types of molecular receptors have been characterised several times. Their presence has been demonstrated in the plasma membrane of stomatal guard cells,53 and it has also been proven that stretch-activated (mechanosensitive) channels translate mechanical stimuli (mechanical energy) into electrical code.46 Probably this mechanism is present also in mechanoreceptor cells of mimosa.
Acknowledgements
We thank Fedora Sutton (South Dakota State University, USA) and Ilona Rácz (Eötvös Loránd University, Hungary) for critical reading of the manuscript.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4743
References
- 1.Ueda M, Takada N, Yamamura S. Molecular approach to the nyctinastic movement of plant controlled by a biological clock. Int J Mol Sci. 2001;2:156–164. [Google Scholar]
- 2.Shimmen T. Involvement of receptor potentials and action potentials on mechano-perception in plants. Aust J Plant Physiol. 2001;28:567–576. [Google Scholar]
- 3.Dutt AK. Vacuoles of pulvinus and the machanism of movement. Nature. 1957;179:254. [Google Scholar]
- 4.Datta M. Vacuoles and movement in pulvinus of Mimosa pudica. Nature. 1957;179:253–254. [Google Scholar]
- 5.Toriyama H, Satô S. Electron microscope observation of the motor cell of Mimosa pudica L. I. Proc Japan Acad. 1968;44:702–706. [Google Scholar]
- 6.Toriyama H, Satô S. Electron microscope observation of the motor cell of Mimosa pudica L. II. Proc Japan Acad. 1968;44:949–953. [Google Scholar]
- 7.Toriyama H, Satô S. On the contents of the central vacuole in the Mimosa motor cell. Cytologia. 1970;36:359–375. [Google Scholar]
- 8.Fleurat-Lessard P, Robin G, Bonmort J, Besse C. Effects of colchicine, vinblasatine, cytochalasine B and phalloidine on seismonastic movements of mimosa pudica laef and on motor cell ultrastructure. J Exp Bot. 1988;39:209–221. [Google Scholar]
- 9.Leopold AC. Many modes of movement. Science. 2000;288:2131–2132. doi: 10.1126/science.288.5474.2131e. [DOI] [PubMed] [Google Scholar]
- 10.Allan RD. Mechanism of the seismonastic reaction in mimosa pudica. Plant Physiol. 1969;44:1101–1107. doi: 10.1104/pp.44.8.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibaoka T. Physiology of rapid movements in higher plants. Annu Rev Plant Physiol. 1969;20:49–73. [Google Scholar]
- 12.Satter RL, Galston AW. Mechanism of control of leaf movements. Annu Rev Plant Physiol. 1981;32:83–110. [Google Scholar]
- 13.Samejima M, Sibaoka T. Changes in the extracellular ion concentration in the main pulvinus of Mimosa pudica during rapid movement and recovery. Plant Cell Physiol. 1980;21:467–479. [Google Scholar]
- 14.Hollins DL, Jaffe MJ. On the role of tannin vacuoles in several nastic leaf responses. Protoplasma. 1997;199:215–222. [Google Scholar]
- 15.Fleurat-Lessard P, Millet B. Ultrastructural features of cortical parenchyma cells (‘Motor Cells’) in stamen filaments of berberis canadensis mill. and tertiary pulvini of Mimosa pudica L. J Exp Bot. 1984;35:1332–1341. [Google Scholar]
- 16.Pickard B. Action potential in higher plants. Bot Rev. 1973;39:172–201. [Google Scholar]
- 17.Fromm J. Control of phloem unloading by action potentials in Mimosa. Physiologia Plantarum. 1991;83:529–533. [Google Scholar]
- 18.Fleurat-Lessard P, Bouché-Pillon S, Leloup C, Bonnenain JL. Distribution and activity of the plasma membrane H+-ATPase in Mimosa pudica L. in relation to ionic fluxes and leaf movements. Plant Physiol. 1997;113:747–754. doi: 10.1104/pp.113.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007;30:249–257. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 20.Ueda M, Yamamura S. Leaf-opening Substance of Mimosa pudica L.; Chemical studies on the other leaf movement of mimosa. Tetrahedron Lett. 1999;40:353–356. [Google Scholar]
- 21.Ueda M, Yamamura S. Leaf-closing Substance of Mimosa pudica L.; Chemical studies on the another leaf movement of mimosa II. Tetrahedron Lett. 1999;40:2981–2984. [Google Scholar]
- 22.Ueda M, Yamamura S. The chemistry of leaf-movement in Mimosa pudica L. Tetrahedron Lett. 1999;55:10937–10948. [Google Scholar]
- 23.Davies E. New functions for signals in plants. New Phytol. 2004;161:607–610. doi: 10.1111/j.1469-8137.2003.01018.x. [DOI] [PubMed] [Google Scholar]
- 24.Zawadzki T, Dziubińnska H, Davies E. Characteristics of action potential generated spontaneously in Helianthus annuus. Physiologia Plantarum. 1995;93:291–297. [Google Scholar]
- 25.Burdon-Sanderson J. Note on the electrical phenomena which accompany irritation of the leaf of Dionaea muscipula in the excited and unexcited states. Proc R Soc (London) 1873;21:491–496. [Google Scholar]
- 26.Sibaoka T. Exitable cells in mimosa. Science. 1962;137:137. doi: 10.1126/science.137.3525.226. [DOI] [PubMed] [Google Scholar]
- 27.Malone M. Wound-induced hydraulic signals and stimulus transmission in Mimosa pudica L. New Phytol. 1994;128:49–56. doi: 10.1111/j.1469-8137.1994.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 28.Braam J. In touch: Plant responses to mechanical stimuli. New Phytol. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 29.Wacke M, Thiel G. Electrically triggered all-or-none Ca2+-liberation during action potential in giant Alga Chara. J Gen Physiol. 2001;118:11–21. doi: 10.1085/jgp.118.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tręebacz K, Simonis W, Schönknecht G. Cytoplasmic Ca2+, K+, Cl−, and NO- activities in the liverwort Conocephalum conicum L. at rest and during action potential. Plant Physiol. 1994;106:1073–1084. doi: 10.1104/pp.106.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kourie JI. Transient Cl− and K+ currents during the action potential in Chara inflate. Plant Physiol. 1994;106:651–660. doi: 10.1104/pp.106.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wildon DC, Thain JF, Minchin PEH, Gubb IR, Reilly AJ, Skipper YD, Doherty HM, O'Donnell PJ, Bowles DJ. Electrical signalling and systemic proteinase inhibitor in wounded plant. Nature. 1992;360:62–65. [Google Scholar]
- 33.Balušska F, Šamaj J, Wojtaszek P, Volkmann D, Menzel D. Cytoskeleton-plasma membrane-cell wall continuum in plants: Emerging links revisited. Plant Physiol. 2003;133:482–491. doi: 10.1104/pp.103.027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davenport R. Glutamate receptors in plants. Annals of Botany. 2002;90:549–557. doi: 10.1093/aob/mcf228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennison KL, Spalding EP. Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol. 2000;124:1511–1514. doi: 10.1104/pp.124.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies E. Action potentials as multifunctional signals in plants: Unifying hypothesis to explain apparently disparate wound response. Plant Cell Environ. 1987;10:623–631. [Google Scholar]
- 37.Herde O, Fuss H, Peña-Cortés H, Fisahn J. Proteinase inhibitor II gene expression induced by electrical stimulation and control of photosynthetic actvity in tomato plants. Plant Cell Physiol. 1995;36:737–742. [Google Scholar]
- 38.Stankovica B, Davies E. Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. FEBS Lett. 1996;390:275–276. doi: 10.1016/0014-5793(96)00672-2. [DOI] [PubMed] [Google Scholar]
- 39.Herde O, Peña-Cortés H, Wasternack C, Willmitzer L, Fisahn J. Electric signaling and Pin2 gene expression on different abiotic stimuli depend on a distinct threshold level of endogenous abscisic acid in several abscisic acid-deficient tomato mutants. Plant Physiol. 1999;119:213–218. doi: 10.1104/pp.119.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisanh J, Herde O, Willmitzer L, Peña-Cortés H. Analysis of the transient increase in cytosolic Ca2+ during the action potential of higher plants with high temporal resolution: Requirement of Ca2+ transients for induction of jasmonic acid biosynthesis and PINII gene expression. Plant Cell Physiol. 2004;45:456–459. doi: 10.1093/pcp/pch054. [DOI] [PubMed] [Google Scholar]
- 41.Fromm J, Hajirezaei M, Wilke I. The biochemical response of electrical signaling in the reproductive system of hibiscus plants. Plant Physiol. 1995;109:375–384. doi: 10.1104/pp.109.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimmen T. Electrical perteption of “Death Message” in Chara: Involvement of turgor pressure. Plant Cell Physiol. 2001;42:366–373. doi: 10.1093/pcp/pce047. [DOI] [PubMed] [Google Scholar]
- 43.Shimmen T. Electrical perteption of “Death Message” in Chara: Analysis of rapid component and ionic process. Plant Cell Physiol. 2002;43:1575–1584. doi: 10.1093/pcp/pcf182. [DOI] [PubMed] [Google Scholar]
- 44.Esau K. On the phloem of Mimosa pudica L. Ann Bot. 1969;34:505–515. [Google Scholar]
- 45.Fleurat-Lessard P, Frangne N, Maeshima M, Ratajczak R, Bonnemain JL, Martinoia E. Increased expression of vacuolar aquaporin and H+-ATPase related to motor cell function in Mimos pudica L. Plant Physiol. 1997;114:827–834. doi: 10.1104/pp.114.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shephard V, Shimmen T, Beilby MJ. Mechanosensory ion Channels in Chara: The influence of cell turgor pressure on touch-activated receptor potentials and action potentials. Aust J Plant Physiol. 2001;28:551–566. [Google Scholar]
- 47.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electronmicroscopy. Jof Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruzin SE. Histochemistry and cytochemistry. Plant Microtechnique and Microscopy. New York: Oxford University Press; 1999. p. 155. [Google Scholar]
- 49.Glover BJ. Differentiation in plant epidermal cells. J of Exp Bot. 2000;51:4197–4505. doi: 10.1093/jexbot/51.344.497. [DOI] [PubMed] [Google Scholar]
- 50.Majore I, Wilhelm B, Marten I. Identification of K+ channels in the plasma membrane of maize subsidiary cells. Plant Cell Physiol. 2002;43:844–852. doi: 10.1093/pcp/pcf104. [DOI] [PubMed] [Google Scholar]
- 51.Raschke K, Fellows MP. Stomatal movement in Zea mays: Shuttle of potassium and chloride between guard cells and subsidiary cells. Planta. 1971;101:296–316. doi: 10.1007/BF00398116. [DOI] [PubMed] [Google Scholar]
- 52.Ward JM, Pei ZM, Schoeder JI. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cosgrove DJ, Hedrich R. Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta. 1991;186:143–153. doi: 10.1007/BF00201510. [DOI] [PubMed] [Google Scholar]