Abstract
Ion homeostasis is essential for plant cell resistance to salt stress. Under salt stress, to avoid cellular damage and nutrient deficiency, plant cells need to maintain adequate K nutrition and a favorable K to Na ratio in the cytosol. Recent observations revealed that both nitric oxide (NO) and hydrogen peroxide (H2O2) act as signaling molecules to regulate K to Na ratio in calluses from Populus euphratica under salt stress. Evidence indicated that NO mediating H2O2 causes salt resistance via the action of plasma membrane H+-ATPase but that activity of plasma membrane NADPH oxidase is dependent on NO. Our study demonstrated the signaling transduction pathway. In this addendum, we proposed a testable hypothesis for NO function in regulation of H2O2 mediating salt resistance.
Key Words: hydrogen peroxide, nitric oxide, signaling molecule, salt resistance
Under salinity conditions, tolerant plant cells achieve ion homeostasis by extruding Na to the external medium and/or compartmentalizing into vacuoles, maintaining K uptake and high K and low Na in the cytosol.1,2 Control of Na movement across the plasma membrane (PM) and tonoplast in order to maintain a low Na concentration in the cytoplasm is a key factor of cellular adaptation to salt stress.3,4 Na transport across the PM is dependent on the electrochemical gradient created by the PM H+-ATPase.5,6 It has been proven that the activity of the PM H+-ATPase is a key index of plant adaptation to salt stress.7 Therefore, the regulation of expression of the PM H+-ATPase may represent an important cellular mechanism for salt resistance. In contrast to our understanding of the regulation of PM H+-ATPase by other factors, the roles of NO and H2O2 act as signals under salt stress have been less known.
Previous studies have shown that both NO and H2O2 function as stress signals in plants, mediating a range of resistance mechanisms in plants under stress conditions.8–10 We have previously shown that NO serves as a signal in inducing salt resistance by increasing the K to Na ratio, which is dependent on the increased PM H+-ATPase activity in calluses from reed.11 Although NO acts as a signal molecule under salt stress and induces salt resistance by increasing PM H+-ATPase activity, our research results also indicated NO can not activate purified PM H+-ATPase activity, at least in vitro. Subsequently, we set out to find the other signal molecules and factors between NO and PM H+-ATPase activity. Since our studies have indicated that NO can not induce salt resistance directly, what roles dose it play in salt resistance in tolerant cells under salt stress? We initially hypothesized ABA or H2O2 might be downstream signal molecules to regulate the activity of PM H+-ATPase. Further results indicated H2O2 content increased greatly under salt stress. Since H2O2 might be the candidate downstream signal molecule, we tested PM H+-ATPase activity and K to Na ratio in calluses by adding H2O2. The results suggested that H2O2 inducing an increased PM H+-ATPase activity resulted in an increased K to Na ratio. Summing up this new assay that allows us to speculate NO maybe regulate the H2O2 generation.
Since H2O2 is involved in downstream signal molecule of NO, PM NADPH oxidase, the main source of H2O2 production, might be the regulated target of NO. We took a pharmacological approach to examine the speculation. The results indicated that PM NADPH oxidase is required for H2O2 accumulation and PM NADPH oxidase activity could attribute to NO in calluses under salt stress. These results also raised another question regarding what concentrations of NO can induce such effects. In our experiments, NO content was induced 1.6 times higher than the control values under salt treatment. We speculated there exists an effective balance point in NO signal system similar to previous reports by Delledonne et al.12 in disease resistance.
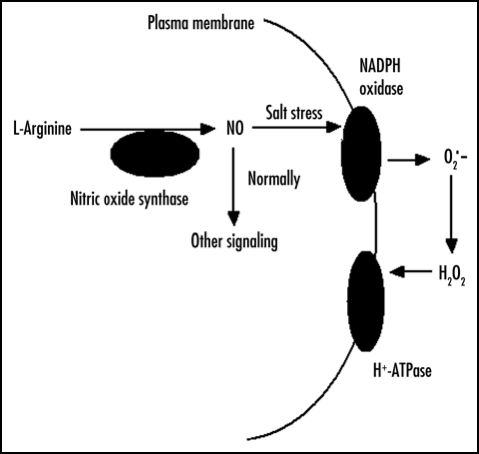
Further research work is required to decipher the mechanism through which NO and H2O2 acts and how K and Na elements uptake might be connected with salt resistance. We would like to propose a simple testable model that accounts for the results reported in this paper (Fig. 1). According to our model, H2O2 rather than NO is the major signaling molecular that mediated directly PM H+-ATPase under salt stress. Normally, NO generated from nitric oxide synthase (NOS) acts as a signal molecule to regulate other mechanisms. Under salt stress, accumulated NO activates PM NADPH oxidase activity. Then, a number of H2O2 is produced from PM NADPH oxidase. The PM H+-ATPase is activated greatly by the accumulated H2O2. Eventually, the transmembrane electrochemical gradient is created and K to Na ratio increases. The model we have proposed here is testable and should provide further insights into salt resistance mechanism regulated by NO and H2O2 signal molecules.
Figure 1.
Hypothetical model for the potential function of NO and H2O2 as signaling molecules in inducing salt resistance. Salt stress activates a signal transduction cascade that leads to the increased activity of PM H+-ATPase, whose expression produces salt resistance. NO is generated by NOS, and H2O2 is produced by NADPH oxidase attributed to NO. The activity of PM H+-ATPase is regulated by H2O2 directly under salt stress. The model is based on the recent results in calluses from P. euphratica12 and those previously reported on the NO function in reed.11
Research on roles of NO and H2O2 under stress conditions in plant is advancing rapidly. Further analysis of salt resistance mechanism with novel technology will certainly increase our knowledge in this field.
Acknowledgements
This work was supported by Gansu Key Laboratory of Crop Genetic & Germplasm Enhancement Science Foundation, Gansu Educational Science Foundation.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4466
References
- 1.Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 2.Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6:1–5. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 3.Niu X, Bressan RA, Hasegawa PM, Pardo JM. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995;109:735–742. doi: 10.1104/pp.109.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morsomme P, Boutry M. The plant plasma membrane H+-ATPase: Structure, function and regulation. Biochim Biophys Acta. 2000;1465:1–16. doi: 10.1016/s0005-2736(00)00128-0. [DOI] [PubMed] [Google Scholar]
- 5.Michelet B, Boutry M. The plasma membrane H+-ATPase: A highly regulated enzyme with multiple physiological functions. Plant Physiol. 1995;108:1–6. doi: 10.1104/pp.108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrano R. Salt tolerance in plants and microorganisms: Toxicity targets and defence responses. Int Rev Cytol. 1996;165:1–52. doi: 10.1016/s0074-7696(08)62219-6. [DOI] [PubMed] [Google Scholar]
- 7.Niu X, Narasimhan ML, Salzman RA, Bressan RA, Hasegawa PM. NaCl regulation of plasma membrane H+-ATPase gene expression in a glycophyte and a halophyte. Plant Physiol. 1993;103:713–718. doi: 10.1104/pp.103.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot. 2002;53:1237–1242. [PubMed] [Google Scholar]
- 9.Wendehenne D, Durner J, Klessig DF. Nitric oxide: A new player in plant signalling and defense responses. Curr Opin Plant Biol. 2004;7:449–455. doi: 10.1016/j.pbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Delledonne M. NO news is good news for plants. Curr Opi Plant Biol. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhao LQ, Zhang F, Guo JK, Yang YL, Li BB, Zhang LX. Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 2004;134:849–857. doi: 10.1104/pp.103.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Wang YP, Yang YL, Wu H, Wang D, Liu JQ. Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Env. 2007;30:775–785. doi: 10.1111/j.1365-3040.2007.01667.x. [DOI] [PubMed] [Google Scholar]