Abstract
Plant growth and development are governed by an intricate web of signaling networks controlled by phytohormones, such as auxin and jasmonic acid. Auxin influences all aspects of plant growth and development, ranging from embryogenesis to root and shoot morphogenesis and organ patterning. Three major groups of auxin-responsive genes have been classified as IAA/AUX, GH3 and SAUR families. Some Group I and II GH3 proteins biochemically function in conjugating amino acids to methyl jasmonate and auxin, respectively. We recently demonstrated that GH3.9, a previously uncharacterized Group II GH3 gene family member, influences primary root growth. Whereas several GH3 family members are transcriptionally induced by auxin, GH3.9 was repressed by exogenous indole-3-acetic acid (IAA) in whole seedlings. GH3.9 promoter::GUS reporter transgenic seedlings showed expression in several tissues, and application of exogenous IAA led to a shift in promoter activity from primary roots to lateral root tips, supporting the hypothesis that GH3.9 maintains auxin homeostasis by redistribution of active auxin pools in roots. GH3.9 mutations influenced both IAA- and methyl jasmonate (MeJA)-mediated root growth inhibition. In this addendum, we expand on a possible role for GH3.9 in crosstalk between auxin and jasmonate signal transduction pathways controlling plant development.
Key Words: amino acid conjugation, Arabidopsis thaliana, auxin, methyl jasmonate, trichomes, roots
Hormones play a critical role in growth and development of multicellular organisms. Plants are no exception to this rule with the cues to organ initiation and development, reproduction, and resistance to biotic and abiotic stresses governed by a fine interplay between phytohormones. Among the well-studied plant hormones, auxin is a major player due to its pleiotropic effects throughout the plant life cycle. Indole-3-acetic acid (IAA) is the prevalent form of naturally occurring auxin and has been implicated in cell division, cell elongation and cell differentiation.1,2 Jasmonic acid (JA) and its derivatives, collectively referred to as jasmonates, comprise another group of plant hormones that are essential for seed germination, root growth, fertility, and defense.3 Isolation of mutants resistant to exogenous JA (e.g., jar1, coi1 and jin mutants) or auxin (e.g., axr mutants) has enhanced our understanding of the mechanisms by which these hormones regulate plant development and biotic and abiotic stress responses.2,4 The physiological effects of these phytohormones are, in part, manifested by altered expression of JA- and auxin-responsive genes.5
Auxin-responsive genes fall into three major families, the so-called AUX/IAA, GH3 and small auxin up RNA (SAUR).6 We recently reported a function for GH3.9 in primary root growth.7 GH3.9 is a Group II GH3 gene postulated to act as an IAA-amido synthetase to conjugate free auxin to amino acids.8,9 Other Group II GH3 gene family members influence primary root growth, hypocotyl elongation, apical dominance, leaf formation and stress responses.10–13 No obvious morphological alterations in the aerial tissues were observed in gh3.9 mutants, possibly due to functional redundancy. However, the gh3.9-1 mutant, GH3.9 RNAi transgenics, and additional T-DNA insertion mutants displayed a long-root phenotype, moderate sensitivity to IAA-mediated root growth inhibition, and moderate resistance to MeJA-mediated root growth inhibition.7 Unlike most other Group II family members, exogenous IAA repressed GH3.9 expression in seedlings. GH3.9 promoter::GUS transgenic seedlings provided greater detail on where GH3.9 might function. The GH3.9 promoter drove expression in root vascular tissues, siliques, seedling root-hypocotyl junctions and mature embryos. Interestingly, exogenous IAA caused increased expression in lateral root tips with a concomitant decrease in primary roots, both sites of auxin biosynthesis.7,14 Polar transport of auxin from root tip to outer cells in root meristem by PIN and PAT proteins is essential for maintaining physiological levels of auxin to ensure proper establishment of root architecture.15 Therefore, GH3.9 may complement auxin transport proteins to regulate active auxin levels in specific root tissues/cell types. In this report, we would like to further comment on GH3.9 possibly affecting plant growth and development regulated by JA-mediated signaling pathways.
GH3.9 in Auxin and Jasmonic Acid Cross Talk?
Biosynthesis, metabolism and transport of plant hormones finely tune gene expression in response to various stimuli. Cross talk between intersecting hormone signaling pathways is the paradigm. For example, expression of JA-responsive genes (JRGs) have been reported to be either repressed or induced by exogenous auxin, supporting both antagonism and synergism between JA- and auxin-mediated signaling pathways.16,17 The extent of this cross talk is exemplified by identification of auxin-resistant1 (axr1) mutants with altered JRG expression in separate genetic screens for mutants resistant to MeJA- and auxin-mediated growth inhibition.16,18 AXR1 encodes an E1 Nedd8/RUB1-activating enzyme, and COI1 encodes an F-box protein that is a subunit of SCFCOI1 E3 ubiquitin ligase.19,20 Therefore, both jasmonate and auxin signal transduction depends on small modifier protein-dependent proteasome-mediated degradation.4
The jar1 mutant, identified in MeJA-mediated root growth inhibition screen, is defective in a Group I GH3 gene family member, JAR1 (GH3.11).9 JAR1 and GH3.9 likely regulate hormone activity by conjugating amino acids to MeJA and auxin, respectively.8,9 JA-isoleucine conjugate levels were significantly reduced in jar1-1¸ while levels of other conjugates, including JA-phenylalanine, were increased, implying that additional JA-conjugating enzymes exist.21 The jar1-1 mutant displayed a short-root phenotype opposite of the gh3.9-1 long-root phenotype on unsupplemented media, prompting us to investigate the response of the gh3.9-1 mutant to MeJA-mediated root growth inhibition. Unexpectedly, we noted similar roles for JAR1 and GH3.9 in MeJA-mediated root growth inhibition, that is both mutants were insensitive to MeJA-mediated root growth inhibition (Fig. 1A).7 Also like jar1-1, fertility was unaffected in gh3.9-1 (data not shown). However, exogenous IAA caused significant changes in GUS activity patterns in roots of GH3.9 promoter::GUS transgenic seedlings, and MeJA had no apparent effect.7 Our observed MeJA-dependent increase in GUS activity in young leaf trichomes may provide another example of integration of auxin and JA-mediated signaling pathways, similar to that performed by AXR1.19,20
Figure 1.
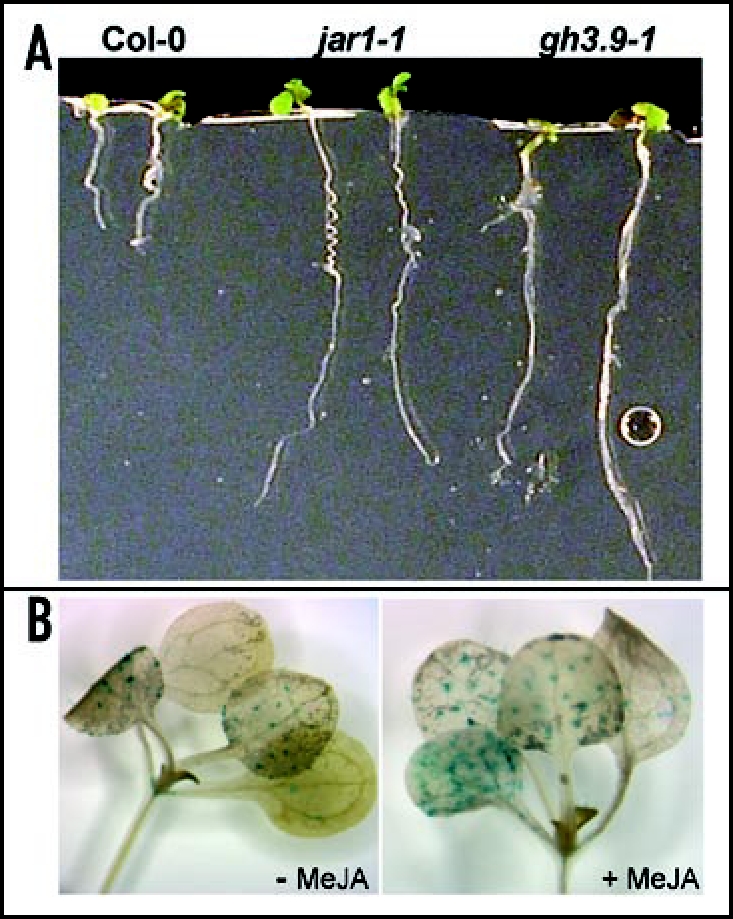
(A) Photographs of representative seedlings (genotypes; wild-type Col-0, mutants jar1-1, and gh3.9-1) after ten days of growth on MS-agar media supplemented with 10 µM MeJA. (B) Photographs of representative transgenic plants harboring a GH3.9 promoter::GUS construct. GH3.9 is expressed in trichomes during leaf development, and exogenous MeJA (10 µM for 60 minutes) enhanced GUS activity in trichomes.
GH3.9 in JA-Mediated Trichome Development?
Jasmonate signaling has been implicated in trichome development by increased JA-dependent trichome density and JRG promoter activity.22,23 To investigate whether GH3.9 expression in trichomes is regulated by JA, GH3.9 promoter::GUS transgenics were treated with exogenous MeJA (10 µM), resulting in a moderate increase in the GUS activity in the trichomes of young leaves (75% GUS positive) compared to untreated seedlings (54% GUS positive; Fig. 1B). Trichome density in the gh3.9-1 mutant was similar to wild-type plants (data not shown) and was also unaffected in the jar1-1 mutant.23 However, given the similar phenotypes related to MeJA-mediated root growth inhibition, it would be interesting to determine whether trichome development is affected in a jar1/gh3.9 double mutant. This double mutant could also be assessed for both IAA- and JA-amino acid conjugate levels and auxin-, JA- or other hormone-responsive gene expression.21,24 These experiments are likely to enhance our understanding of the physiological functions of GH3.9 and JAR1, the importance of hormone conjugation to amino acids and the intricacy of hormone cross talk in plant development.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4498
References
- 1.Teale WD, Paponov IA, Palme K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 2.Woodward AW, Bartel B. Auxin: Regulation, action, and interaction. Ann Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasternack C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007 doi: 10.1093/aob/mcm079. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzo O, Solano R. Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol. 2005;8:532–540. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Nemhauser JL, Hong F, Chory J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell. 2006;126:467–475. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Hagen G, Guilfoyle T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol Biol. 2002;49:373–385. [PubMed] [Google Scholar]
- 7.Khan S, Stone JM. Arabidopsis thaliana GH3.9 influences primary root growth. Planta. 2007;226:21–34. doi: 10.1007/s00425-006-0462-2. [DOI] [PubMed] [Google Scholar]
- 8.Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staswick PE, Tiryaki I, Rowe ML. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakazawa M, Yabe N, Ichikawa T, Yamamoto YY, Yoshizumi T, Hasunuma K, Matsui M. DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J. 2001;25:213–221. doi: 10.1046/j.1365-313x.2001.00957.x. [DOI] [PubMed] [Google Scholar]
- 11.Takase T, Nakazawa M, Ishikawa A, Kawashima M, Ichikawa T, Takahashi N, Shimada H, Manabe K, Matsui M. ydk1-D, an auxin-responsive GH3 mutant that is involved in hypocotyl and root elongation. Plant J. 2004;37:471–483. doi: 10.1046/j.1365-313x.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- 12.Takase T, Nakazawa M, Ishikawa A, Manabe K, Matsui M. DFL2, a new member of the Arabidopsis GH3 gene family, is involved in red light-specific hypocotyl elongation. Plant Cell Physiol. 2003;44:1071–1080. doi: 10.1093/pcp/pcg130. [DOI] [PubMed] [Google Scholar]
- 13.Park JE, Park JY, Kim YS, Staswick PE, Jeon J, Yun J, Kim SY, Kim J, Lee YH, Park CM. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem. 2007;282:10036–10046. doi: 10.1074/jbc.M610524200. [DOI] [PubMed] [Google Scholar]
- 14.Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell. 2005;17:1090–1104. doi: 10.1105/tpc.104.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming AJ. Plant signalling: The inexorable rise of auxin. Trends Cell Biol. 2006;16:397–402. doi: 10.1016/j.tcb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Tiryaki I, Staswick PE. An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol. 2002;130:887–894. doi: 10.1104/pp.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeWald DB, Sadka A, Mullet JE. Sucrose modulation of soybean vsp gene expression is inhibited by auxin. Plant Physiol. 1994;104:439–444. doi: 10.1104/pp.104.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwechheimer C, Serino G, Deng XW. Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell. 2002;14:2553–2563. doi: 10.1105/tpc.003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng S, Ma L, Wang X, Xie D, Dinesh-Kumar SP, Wei N, Deng XW. The COP9 signalosome interacts physically with SCFCOI1 and modulates jasmonate responses. Plant Cell. 2003;15:1083–1094. doi: 10.1105/tpc.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason HS, DeWald DB, Mullet JE. Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell. 1993;5:241–251. doi: 10.1105/tpc.5.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traw MB, Bergelson J. Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol. 2003;133:1367–1375. doi: 10.1104/pp.103.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis C, Turner JG. A conditionally fertile coi1 allele indicates cross-talk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings. Planta. 2002;215:549–556. doi: 10.1007/s00425-002-0787-4. [DOI] [PubMed] [Google Scholar]


