Abstract
We have recently identified cytokinin as an important xylem-carried signal involved in the photosynthetic acclimation of plants to light gradients in dense canopies. Lower leaves become shaded in a dense canopy and consequently have reduced transpiration rates. our measurements have shown that this results in a reduced delivery of cytokinins carried in the transpiration stream to shaded leaves, as compared to light-exposed leaves. Cytokinins are involved in the regulation of photosynthetic acclimation to the light gradient by stimulating the expression of photosynthetic enzymes in light-exposed leaves. In shaded leaves, the low delivery rate of cytokinin leads to reduced photosynthetic capacity and ultimately senescence. We show evidence for this role of cytokinin, as part of a complex of signaling pathways where other regulatory mechanisms are also involved. A model is presented depicting the regulation of photosynthetic acclimation by cytokinin delivery to leaves dependent on the irradiance they receive.
Key Words: canopy light gradient, transpiration, photosynthetic acclimation, cytokinin, nitrate, systemic signaling
Introduction
Plants in dense leaf canopies distribute their photosynthetic capacity among leaves in parallel to the vertical light gradient. This acclimation to canopy light gradients improves photosynthetic light use efficiency at the whole plant level and contributes to fitness in dense stands,1–3 as was recently confirmed experimentally.1 Therefore, plants must posses a mechanism by which the canopy light gradient is perceived and the signal is transduced to result in appropriate regulation of photosynthetic enzymes. In the April issue of Plant Physiology, we have shown that cytokinin transported in the transpiration stream plays an important part in this mechanism.
Previous work has demonstrated that the gradient in leaf transpiration rates, which parallels the vertical light gradient in canopies (Fig. 1), is involved in regulating photosynthetic acclimation.4–6 A reduced transpiration rate of shaded leaves in lower layers of a dense canopy implies that any compound carried in the xylem sap is a potential candidate signal for irradiance, as its delivery rate to leaves varies with the irradiance received. When the transpiration rate of attached, light-exposed leaves was reduced by surrounding it with humid air, several aspects of shading could be mimicked.
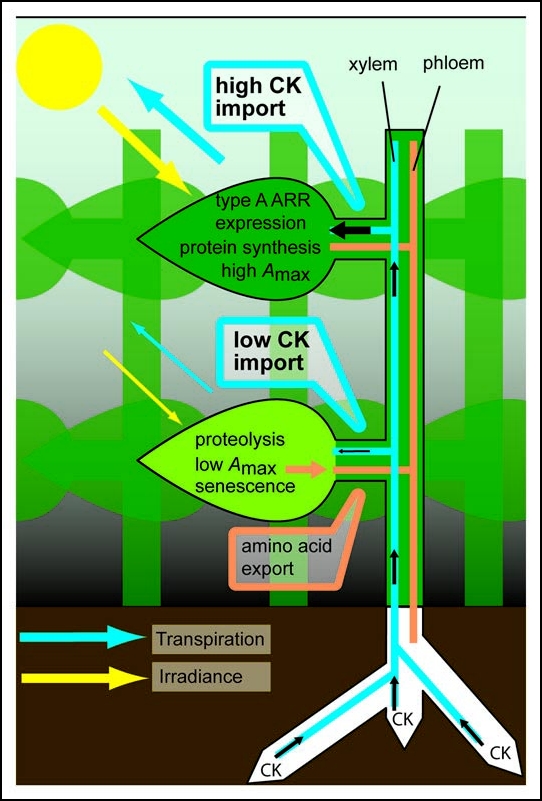
Figure 1.
Model depicting the proposed delivery of root-borne cytokinin (CK) to leaves dependent on incident irradiance. In a dense canopy, lower leaves are shaded so their stomatal conductance and transpiration rate are reduced compared to light-exposed, upper leaves. Consequently, shaded leaves import less cytokinins, which are synthesized in the roots and transported via the xylem. In light-exposed leaves cytokinin stimulates the expression of (homologs of) type A ARRs and photosynthetic enzymes, contributing to high photosynthetic capacity (Amax). On the contrary, low cytokinin import in shaded leaves leads to reduced Amax, enhanced protease activity and ultimately senescence. Amino acids are exported from shaded leaves via the phloem and may be redistributed to upper leaves.
A likely candidate for the xylem-carried signal is cytokinin, given its role in stimulating the synthesis of photosynthetic enzymes7–9 and inhibiting protease activity10 and senescence.11,12 Indeed, cytokinin concentrations in canopies and in shaded leaves varied consistently with this model and application of cytokinin to shaded leaves on a plant remaining in high light rescued the shading effect to a large extent.5
In this study we set out to further test the hypothesis that the cytokinin delivery rate to leaves is a canopy density signal leading to photosynthetic acclimation. We used the rapidly increasing knowledge on cytokinin signal transduction and metabolism. Canopy processes were investigated with tobacco as a model plant and Arabidopsis provided the necessary molecular tools.
Cytokinin Delivery
A vital piece of evidence in support of our model was obtained by measuring cytokinins in the xylem sap of shaded versus light-exposed leaves on the same tobacco plant. A computer-controlled system to pressurize roots was used that allowed the sampling of xylem sap from a transpiring plant.13 Small incisions were made in the midrib of the leaves and about 10% of the xylem sap delivered to the leaves was collected. Analysis using liquid chromatography and tandem mass spectrometry revealed that the concentration of iP-type cytokinins was similar in the xylem sap delivered to either the shaded or the light-exposed leaf on the same plant. Since shaded leaves have far lower transpiration rates and import is the product of concentration and transpiration rate, we concluded that shaded leaves indeed import less cytokinin. Accordingly, shaded leaves and leaves kept in humid air had lower cytokinin concentrations compared to leaves remaining in high light at normal humidity, particularly those of the bioactive Zeatin type were reduced.
Multiple Signaling Pathways Involved
Another candidate signaling molecule transported in the xylem is nitrate, which is both a resource for photosynthetic proteins and acts as a signal stimulating its own assimilation in leaves and cytokinin production in roots.14,15 Although nitrate could act in a manner similar to the one we propose for cytokinin, our finding of much higher concentrations in shaded leaves argues against such a function. Most of the large proportion of nitrate delivered to light-exposed leaves is likely to be assimilated and incorporated into proteins so nitrate does not accumulate there, whereas nitrate is utilized to a much lesser extent in shaded leaves where it accumulates.
Photoreceptors are involved in the perception of canopy density with respect to the morphological aspects of shade avoidance.16 Phytochrome was shown to be also involved in the induction of senescence of lower canopy leaves which receive a reduced red to far-red light ratio.17,18 However, there is no evidence that other photoreceptors are involved in the photosynthetic acclimation to canopy light gradients. All photoreceptor mutants tested so far retained their ability to reduce photosynthetic capacity in response to shading and form distinct sun or shade leaves.19 We observed the same in a survey of a large number of photoreceptor mutants of the phytochrome, cryptochrome and phototropin families, including double and triple mutants.20 In the same survey, various cytokinin receptor mutants also showed reduction in photosynthetic capacity in response to shading. These data indicate that neither photoreceptors nor cytokinin are fully responsible on their own but rather that multiple signaling routes are involved and have partially overlapping functions in photosynthetic acclimation to light gradients.
Lower Cytokinin Activity in Shaded Leaves Decreased the Expression of Photosynthetic Genes
In our study we have made use of cytokinin-responsive genes belonging to the type A Arabidopsis Response Regulators (ARRs).21,22 Monitoring their transcript levels provides a measure of cytokinin activity at the level of gene transcript regulation. When the transpiration of an Arabidopsis leaf in the light was experimentally reduced, we observed reduced expression of ARR7 and ARR16, corresponding well with reduced concentrations of several active cytokinins. Reduced transpiration rate proved to be sufficient to decrease photosynthetic capacity, transcript levels of the rbcS gene encoding the small subunit of Rubisco and it led to accelerated senescence. Furthermore, exogenously applied cytokinin or localized overproduction of cytokinin rescued the effects of partial shade or humid air treatment. These data provide further evidence for an important role of cytokinins in regulating photosynthetic acclimation of plants to light gradients in canopies. The figure depicts a schematic overview of this mechanism.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4573
References
- 1.Boonman A, Anten NPR, Dueck TA, Jordi WJRM, van der Werf A, Voesenek LACJ, Pons TL. Functional significance of shade-induced leaf senescence: An experimental test using transgenic tobacco. Amer Nat. 2006;168:597–607. doi: 10.1086/508633. [DOI] [PubMed] [Google Scholar]
- 2.Hirose T. Development of the Monsi-Saeki theory on canopy structure and function. Ann Bot. 2005;95:483–494. doi: 10.1093/aob/mci047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anten NPR. Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Ann Bot. 2005;95:495–506. doi: 10.1093/aob/mci048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pons TL, Bergkotte M. Nitrogen reallocation in response to partial shading of a plant: Possible mechanisms. Physiol Plant. 1996;98:571–577. [Google Scholar]
- 5.Pons TL, Jordi W, Kuiper D. Acclimation of plants to light gradients in leaf canopies; evidence for a possible role for cytokinins transported in the transpiration stream. J Exp Bot. 2001;52:1–12. doi: 10.1093/jexbot/52.360.1563. [DOI] [PubMed] [Google Scholar]
- 6.Pons TL, Jordi W. Induction of leaf senescence and shade acclimation in leaf canopies- Variation with leaf longevity. In: Lambers H, Poorter H, van Vuuren MMI, editors. Inherent variation in plant growth; Physiological mechanisms and ecological consequences. Leiden, The Netherlands: Backhuys Publishers; 1998. pp. 121–137. [Google Scholar]
- 7.Chory J, Reinecke D, Sim S, Washburn T, Brenner M. A role for cytokinins in de-etiolation in Arabidopsis (det mutants have an altered response to cytokinins) Plant Physiol. 1994;104:339–347. doi: 10.1104/pp.104.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores S, Tobin EM. Cytokinin modulation of LHCP mRNA levels: The involvement of post-transcriptional regulation. Plant Mol Biol. 1988;11:409–415. doi: 10.1007/BF00039021. [DOI] [PubMed] [Google Scholar]
- 9.Kusnetsov VV, Oelmüller R, Sarwat MI, Porfirova SA, Cherepneva GN, Hermann RG, Kulaeva ON. Cytokinins, abscisic acid and light affect accumulation of chloroplast proteins in Lupinus luteus cotyledons without notable effect on steady-state mRNA levels. Planta. 1994;194:318–327. [Google Scholar]
- 10.Li Q, Bettany AJE, Donnison I, Griffiths CM, Thomas H, Scott IM. Characterisation of a cysteine protease cDNA from Lolium multiflorum leaves and its expression during senescence and cytokinin treatment. Biochim Biophys Acta. 2000;1492:233–236. doi: 10.1016/s0167-4781(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 11.Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- 12.Jordi W, Schapendonk A, Davelaar E, Stoopen GM, Pot CS, De Visser R, Van Rhijn JA, Gan S, Amasino RM. Increased cytokinin levels in transgenic PSAG12-IPT tobacco plants have large direct and indirect effects on leaf senescence, photosynthesis and N partitioning. Plant Cell Environ. 2000;23:279–289. [Google Scholar]
- 13.Schurr U, Schulze ED. The concentration of xylem sap constituents in root exudate, and in sap from intact, transpiring castor bean plants (Ricinus communis L.) Plant Cell Environ. 1995;18:409–420. [Google Scholar]
- 14.Stitt M. Nitrate regulation of metabolism and growth. Curr Opin Plant Biol. 1999;2:178–186. doi: 10.1016/S1369-5266(99)80033-8. [DOI] [PubMed] [Google Scholar]
- 15.Takei K, Sakakibara H, Taniguchi M, Sugiyama T. Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: Implication of cytokinin species that induces gene expression of maize response regulator. Plant Cell Physiol. 2001;42:85–93. doi: 10.1093/pcp/pce009. [DOI] [PubMed] [Google Scholar]
- 16.Ballaré CL. Keeping up with the neighbours: Phytochrome sensing and other signalling mechanisms. Trends Plant Sci. 1999;4:97–102. doi: 10.1016/s1360-1385(99)01383-7. [DOI] [PubMed] [Google Scholar]
- 17.Pons TL, de Jong-van Berkel Y. Species-specific variation in the importance of the spectral quality gradient in canopies as a signal for photosynthetic resource partitioning. Ann Bot. 2004;94:725–732. doi: 10.1093/aob/mch197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousseaux MC, Hall AJ, Sánchez RA. Basal leaf senescence in a sunflower (Helianthus annuus) canopy: Responses to increased R/FR ratio. Physiol Plant. 2000;110:477–482. [Google Scholar]
- 19.Terashima I, Araya T, Miyaza SI, Sone K, Yano S. Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: An eco-developmental treatise. Ann Bot. 2005;95:507–519. doi: 10.1093/aob/mci049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boonman A. Plant acclimation to the light gradient in canopies; functional significance and regulation by cytokinin. Utrecht, The Netherlands: Utrecht University; PhD Thesis. [Google Scholar]
- 21.D'Agostino IB, Deruère J, Kieber JJ. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 2000;124:1706–1717. doi: 10.1104/pp.124.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiba T, Yamada H, Mizuno T. Characterization of the ARR15 and ARR16 response regulators with special reference to the cytokinin signaling pathway mediated by the AHK4 histidine kinase in roots of Arabidopsis thaliana. Plant Cell Physiol. 2002;43:1059–1066. doi: 10.1093/pcp/pcf121. [DOI] [PubMed] [Google Scholar]