Abstract
Seed dormancy and germination are complex physiological processes usually under hormonal control. Germination of seeds from many plants including switchgrass, are inhibited by ABA and promoted by NO or ROS. However, ABA apparently requires both ROS and NO as intermediates in its action, with ROS produced by membrane-bound NADPH-oxidases responsive to ABA. In switchgrass seeds, externally supplied hydrogen peroxide (ROS), but not NO will overcome ABA-imposed inhibition of germination. Stimulation of germination by external ROS can be partially blocked by NO-scavengers, suggesting that NO is required for seed germination in switchgrass as well as for ABA-induced inhibition of germination. Collectively, these data suggest that multiple mechanisms might be required to sense and respond to varying levels of ABA, NO and ROS in switchgrass seeds.
Key Words: switchgrass, seed germination, ROS, hydrogen peroxide, ABA, nitric oxide
ABA Action Requires ROS and NO
Elegant work with several plants has documented the requirement of ROS/hydrogen peroxide and NO during stomatal movement, seed germination and leaf senescence.1–7 To bring about its action, ABA requires both hydrogen peroxide and NO and is probably initiated when ABA binds with both membrane bound and soluble receptors precipitating downstream signaling.8–10 An integral part of this signaling mechanism appears to involve a number of proteins,11–15 including protein kinases16 and protein phosphatases,17 and one or more members of the reactive-burst oxidases.18–19 Blocking NO or peroxide through the use of scavengers generally blocks ABA action5,20–21 indicating a causal relationship between these molecules. Recently, Zhang et al.21 have shown that ABA-induced peroxide production occurs upstream of NO production, and at least in maize leaves, hydrogen peroxide was required for the generation of NO.
Seed Germination is Impacted by NO, ROS and ABA
Seed dormancy in many species is principally imposed by ABA22 and broken by NO,23–25 cyanide26 and by hydrogen peroxide.27 These studies indicate that in seeds, high levels of NO and/or ROS are required for germination. Data from barley and Arabidposis1,28 further show that the aleurone layer is the dominant site for NO production and perception during seed germination.
Switchgrass Seed Germination
Switchgrass seed germination responds positively to external sources of NO, cyanide and ROS.29–30 These chemical treatments overcame residual dormancy and significantly stimulated germination. A chemical scavenger of NO effectively blocked the enhancement of germination induced by NO or ROS suggesting that ROS action is at least partially perceived through NO.30 The aleurone appeared to be the dominant site of NO production in switchgrass as judged by confocal microscopy, and aleurone activation was enhanced in seeds treated with hydrogen peroxide.30 Seed germination was blocked by ABA as well as by diphenyleneiodonium (DPI) an inhibitor of Rbohs suggesting that internal generation of ROS by Rbohs was needed for switchgrass seed germination, but could occur independently of ABA-induced peroxide formation. Externally supplied H2O2, but not NO overcame ABA-imposed inhibition of switchgrass seed germination, implying that high seed ROS or a process responsive to high ROS levels could override ABA responsive anti-germinative pathways in switchgrass.29–30
A Potential for Two NO-Sensors in Switchgrass Seeds
Data described above would favor the presence of at least two NO-sensors in seeds as shown in Figure 1. This model assumes that NO is an obligate part of ABA action in plants.5,21 In dormant and imbibed seeds continued presence of ABA blocks germination suggesting that NO perception would require one or more sensor that responds to low NO levels. Upon treating seeds with chemicals that increase endogenous NO levels6,23–26,29 germination is stimulated suggesting that one or more NO-sensors might be required that respond to higher or a more constant presence of NO resulting in a different physiological output (for example germination) as compared to seeds with high ABA/low NO levels. In switchgrass, high internal NO level cannot overcome ABA-induced inhibition29 implying that ABA can limit output 2 (Fig. 1). In contrast, externally supplied ROS in the form of H2O2 stimulates NO production in the aleurone, significantly enhances germination and can overcome ABA-imposed inhibition of switchgrass seed germination.30 A portion of this stimulation of seed germination by H2O2 is blocked by a scavenger of NO indicating that external ROS also has to act partially through the high NO-sensor(s) pathway (Output 2; Fig. 1). Studies with DPI30 provide some evidence for the involvement of Rbohs during switchgrass seed germination, and these have been shown as Rboh I and Rboh II in Figure 1. It has been documented that ABA requires the generation of hydrogen peroxide as well as specific Rbohs.5,20 In switchgrass seeds DPI partially inhibited germination and NADPH-oxidase activity in seed extracts implying that ROS produced by Rbohs are potentially important to the germination process. One could postulate that different or the same Rbohs could participate in pro-germinative or anti-germinative cascades depending on other interacting proteins and the relative metabolic and hormonal status of the seed. Finally, high external ROS (added H2O2) blocks ABA-responsive mechanisms, stimulates high NO levels predominantly in the aleurone and appears to also function partially through an NO-independent sensing mechanism (output 3). Our model also suggests that differential tissue distribution of sensors could result in multiple outcomes in response to ABA, NO and ROS. Although Figure 1 is speculative to some degree, it allows formulation of future experiments to delineate key components involved in switchgrass seed germination and dormancy.
Figure 1.
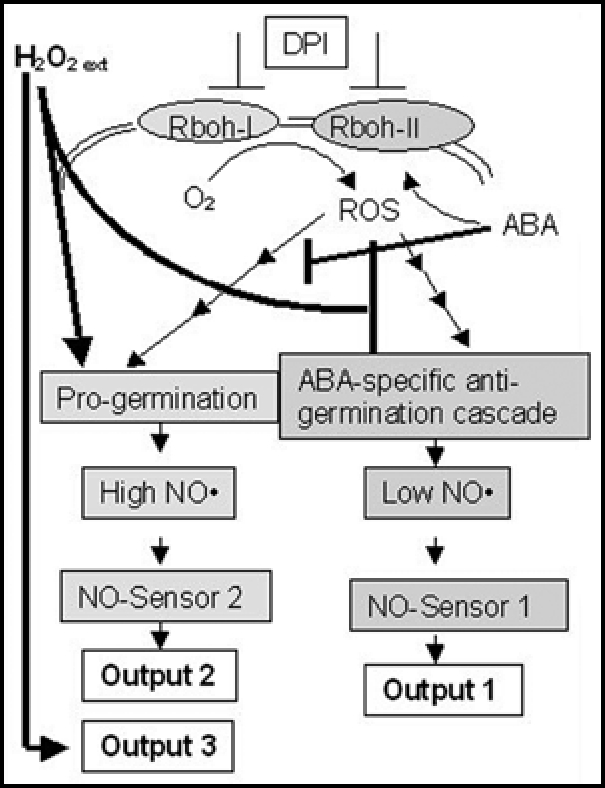
A potential for two NO-sensors in switchgrass seeds. DPI, diphenyleneiodonium; Rboh, reactive burst oxidases.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4575
References
- 1.Bethke PC, Libourel IG, Aoyama N, Chung YY, Still DW, Jones RL. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143:1173–1188. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inze D, Delledonne M, Van Breusegem F. Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol. 2006;141:404–411. doi: 10.1104/pp.106.078444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Bot. 2002;53:1237–1247. [PubMed] [Google Scholar]
- 4.Ross C, Kupper FC, Jacobs RS. Involvement of reactive oxygen species and reactive nitrogen species in the wound response of Dasycladus vermicularis. Chem Biol. 2006;13:353–364. doi: 10.1016/j.chembiol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 6.Bethke PC, Libourel IG, Jones RL. Nitric oxide reduces seed dormancy in Arabidopsis. J Exp Bot. 2006;57:517–526. doi: 10.1093/jxb/erj060. [DOI] [PubMed] [Google Scholar]
- 7.Hung KT, Kao CH. Hydrogen peroxide is necessary for abscisic acid-induced senescence of rice leaves. J Plant Physiol. 2004;161:1347–1357. doi: 10.1016/j.jplph.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 9.Shen YY, Wang XF, Wu FQ, Du SY, Cao Z, Shang Y, Wang XL, Peng CC, Yu XC, Zhu SY, Fan RC, Xu YH, Zhang DP. The Mg-chelatase H subunit is an abscisic acid receptor. Nature. 2006;443:823–826. doi: 10.1038/nature05176. [DOI] [PubMed] [Google Scholar]
- 10.Razem FA, El-Kereamy A, Abrams SR, Hill RD. The RNA-binding protein FCA is an abscisic acid receptor. Nature. 2006;439:290–294. doi: 10.1038/nature04373. [DOI] [PubMed] [Google Scholar]
- 11.Warpeha KM, Upadhyay S, Yeh J, Adamiak J, Hawkins SI, Lapik YR, Anderson MB, Kaufman LS. The GCR1, GPA1, PRN1, NF-Y signal chain mediates both blue light and abscisic acid responses in Arabidopsis. Plant Physiol. 2007;143:1590–1600. doi: 10.1104/pp.106.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 2006;18:2749–2766. doi: 10.1105/tpc.106.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng X, Phillips J, Brautigam A, Engstrom P, Johannesson H, Ouwerkerk PB, Ruberti I, Salinas J, Vera P, Iannacone R, Meijer AH, Bartels D. A homeodomain leucine zipper gene from Craterostigma plantagineum regulates abscisic acid responsive gene expression and physiological responses. Plant Mol Biol. 2006;61:469–489. doi: 10.1007/s11103-006-0023-x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Yu L, Zhang Y, Wang X. Phospholipase D in the signaling networks of plant response to abscisic acid and reactive oxygen species. Biochim Biophys Acta. 2005;1736:1–9. doi: 10.1016/j.bbalip.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Fujii H, Verslues PE, Zhu JK. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell. 2007;19:485–494. doi: 10.1105/tpc.106.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura N, Yoshida T, Kitahata N, Asami T, Shinozaki K, Hirayama T. ABA-Hypersensitive Germination1 encodes a protein phosphatase 2C, an essential component of abscisic acid signaling in Arabidopsis seed. Plant J. 2007;50:935–949. doi: 10.1111/j.1365-313X.2007.03107.x. [DOI] [PubMed] [Google Scholar]
- 18.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Desikan R, Griffiths R, Hancock J, Neill S. A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang A, Jiang M, Zhang J, Ding H, Xu S, Hu X, Tan M. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007;175:36–50. doi: 10.1111/j.1469-8137.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- 22.Gubler F, Millar AA, Jacobsen JV. Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol. 2005;8:183–187. doi: 10.1016/j.pbi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Beligni MV, Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–221. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- 24.Bethke PC, Gubler F, Jacobsen JV, Jones RL. Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta. 2004;219:847–855. doi: 10.1007/s00425-004-1282-x. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Liu X, Ajmal Khan M, Yamaguchi S. The effect of plant growth regulators, nitric oxide, nitrate, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions. J Plant Res. 2005;118:207–214. doi: 10.1007/s10265-005-0212-8. [DOI] [PubMed] [Google Scholar]
- 26.Bethke PC, Libourel IG, Reinohl V, Jones RL. Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta. 2006;223:805–812. doi: 10.1007/s00425-005-0116-9. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa K, Iwabuchi M. A mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant Cell Physiol. 2001;42:286–291. doi: 10.1093/pcp/pce032. [DOI] [PubMed] [Google Scholar]
- 28.Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL. Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 2002;129:1642–1650. doi: 10.1104/pp.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarath G, Bethke PC, Jones R, Baird LM, Hou G, Mitchell RB. Nitric oxide accelerates seed germination in warm-season grasses. Planta. 2006;223:1154–1164. doi: 10.1007/s00425-005-0162-3. [DOI] [PubMed] [Google Scholar]
- 30.Sarath G, Hou G, Baird LM, Mitchell RB. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C(4)-grasses. Planta. 2007;226:697–708. doi: 10.1007/s00425-007-0517-z. [DOI] [PubMed] [Google Scholar]


