Abstract
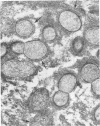
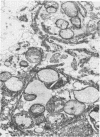
Light and electron microscopy with histochemical staining were used to estimate exopolysaccharide production by strains of viridans streptococci recovered from patients with endocarditis. Six strains were selected for study because they represented a wide range of in vitro polysaccharide production. By light microscopy, there was good agreement between three polysaccharide stains (ruthenium red, periodic acid-Schiff and calcifluor white) in the amount of glycocalyx produced, which ranged from minimal (0 to 1+) to maximal amounts (4+). Two strains selected for minimal (strain 1) and maximal (strain 6) in vitro exopolysaccharide production were studied after we used them to experimentally infect cardiac vegetations. Glycocalyx could be demonstrated surrounding organisms in cardiac vegetations, and the relative amounts produced were similar to those seen in vitro. Vegetations formed by glycocalyx-producing strains were also larger than those formed by glycocalyx-deficient strains. Viridans group streptococci which produce exopolysaccharide in vitro also do so within cardiac vegetations. The relationship of exopolysaccharide production to maintenance of endocardial infection is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S., Mitchell M. Immunologic investigations of mucoid strains of Pseudomonas aeruginosa: comparison of susceptibility to opsonic antibody in mucoid and nonmucoid strains. J Infect Dis. 1980 Feb;141(2):238–247. doi: 10.1093/infdis/141.2.238. [DOI] [PubMed] [Google Scholar]
- Caputy G. G., Costerton J. W. Morphological examination of the glycocalyces of Staphylococcus aureus strains Wiley and Smith. Infect Immun. 1982 May;36(2):759–767. doi: 10.1128/iai.36.2.759-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R., Acres S. D., Costerton J. W. Use of specific antibody to demonstrate glycocalyx, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrum-fed calves. Infect Immun. 1982 Sep;37(3):1170–1180. doi: 10.1128/iai.37.3.1170-1180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Irvin R. T., Costerton J. W. Autochthonous and pathogenic colonization of animal tissues by bacteria. Can J Microbiol. 1981 May;27(5):461–490. doi: 10.1139/m81-071. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. II. Survival of a bacteria in endocardial vegetations. Br J Exp Pathol. 1972 Feb;53(1):50–53. [PMC free article] [PubMed] [Google Scholar]
- Garrison P. K., Freedman L. R. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970 Jun;42(6):394–410. [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Sande M. A. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect Immun. 1974 Dec;10(6):1433–1438. doi: 10.1128/iai.10.6.1433-1438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., McCully M. E. The use of an optical brightener in the study of plant structure. Stain Technol. 1975 Sep;50(5):319–329. doi: 10.3109/10520297509117082. [DOI] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Mackie E. B., Brown K. N., Lam J., Costerton J. W. Morphological stabilization of capsules of group B streptococci, types Ia, Ib, II, and III, with specific antibody. J Bacteriol. 1979 May;138(2):609–617. doi: 10.1128/jb.138.2.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Prolonged survival of Serratia marcescens in chlorhexidine. Appl Environ Microbiol. 1981 Dec;42(6):1093–1102. doi: 10.1128/aem.42.6.1093-1102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Lam J., Costerton J. W. Bacterial adhesion to uroepithelial cells: a morphologic study. J Infect Dis. 1980 Aug;142(2):239–246. doi: 10.1093/infdis/142.2.239. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- McCoy W. F., Bryers J. D., Robbins J., Costerton J. W. Observations of fouling biofilm formation. Can J Microbiol. 1981 Sep;27(9):910–917. doi: 10.1139/m81-143. [DOI] [PubMed] [Google Scholar]
- Pulliam L., Porschen R. K., Hadley W. K. Biochemical properties of CO2-dependent streptococci. J Clin Microbiol. 1980 Jul;12(1):27–31. doi: 10.1128/jcm.12.1.27-31.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J Clin Invest. 1978 Oct;62(4):805–814. doi: 10.1172/JCI109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Effects of molecular weight of dextran on the adherence of Streptococcus sanguis to damaged heart valves. Infect Immun. 1980 Jul;29(1):1–7. doi: 10.1128/iai.29.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thörig L., Thompson J., Eulderink F., Emeis J. J., Van Furth R. Effects of monocytopenia and anticoagulation in experimental Streptococcus sanguis endocarditis. Br J Exp Pathol. 1980 Feb;61(1):108–116. [PMC free article] [PubMed] [Google Scholar]
- Yersin B. R., Glauser M. P., Freedman L. R. Effect of nitrogen mustard on natural history of right-sided streptococcal endocarditis in rabbits: role for cellular host defenses. Infect Immun. 1982 Jan;35(1):320–325. doi: 10.1128/iai.35.1.320-325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]