Abstract
More than 10% of the plant-specific NAC (NAM, ATAF1/2, CUC2) transcription factors have been predicted to have alpha-helical transmembrane (TM) domain in their C-terminal regions, among which at least three members have been proven to be membrane-associated and play a role in cell cycle control and stress responses. These observations suggest that membrane-mediated regulation would be an important molecular mechanism mediating rapid transcriptional responses to internal and external stimuli in plants. Recently, we showed that a salt-responsive NTL (NTM1-Like's) transcription factor NTL8 is localized primarily in plasma membranes as dormant form and subsequently processed into transcriptionally active, nuclear form. Overexpression of an active NTL8 form exhibited delayed flowering as well as reduced growth with small curled leaves. Consistent with this, expression of FLOWERING LOCUS T (FT) and its downstream genes was significantly reduced in the transgenic plants. Furthermore, FT was notably repressed by high salt. These results indicate that NTL8 mediates salt-responsive flowering via FT in Arabidopsis and that membrane-mediated transcription regulation underlies the salt signaling in mediating flowering initiation.
Key Words: Arabidopsis, flowering time, flowering locus T (FT), membrane-bound transcription factor, NAC, salt stress
In a fairy story, a sleeping princess was awakened by the kiss from a prince. Recent studies demonstrate that a similar episode is occurring in the cell. A group of transcription factors is tethered to cellular membranes as inactive forms (sleeping). When it is kissed by the ubiquitin/proteasome machinery (RUP) or by membrane-associated proteases (RIP), an active form is released from the membranes and translocated into the nucleus, where it turns on target genes.1 This activation mechanism guarantees rapid transcriptional response to internal and environmental changes in yeast, animals and plants.2–4 Recent studies have revealed that a considerable portion of the NAC transcription factors are associated with the intracellular membranes.5,6 These NAC members have been collectively termed NTLs for NTM1-Like's.7
NTL8, one of the NTLs, is consisted of 335 amino acids with a predicted TM domain in its far C-terminal region.6,8 To confirm the membrane localization of NTL8, a full size NTL8 (8F) and a truncated NTL8 (8ΔC) lacking the TM domain was translationally fused with GFP, and the fusion constructs were transiently expressed in onion epidermal cells. Whereas the 8F signal was predominantly detected in association with the plasma membranes, the 8ΔC signal was exclusively located in the nucleus, confirming the membrane association of NTL8.
The 8F-overproducing transgenic plants (35S::8F) exhibited a moderate degree of growth reduction but with apparently normal leaf morphology. In contrast, those transformed with the 8ΔC construct (35S::8ΔC) exhibited two distinct phenotypes with severe morphological and developmental alterations. One line (35S::8ΔC-1) was late flowering with normal leaf morphology and the other line (35S::8ΔC-2) exhibited severe phenotypic changes, including reduced growth with small, curled leaves, in addition to late flowering, which looks like a plant grown under stress conditions. These observations demonstrate that membrane release is essential for NTL8 function, as has been proven with NTM1.5 It is also envisaged that NTL8 might be related to plant stress responses. Meanwhile, a knockout ntl8-1 mutant did not exhibit any discernible phenotypic changes, although it was slightly different from wild type plants in lateral root growth and flowering time. This may be due to a functional redundancy among the NTL members. Although primary root growth was normal in the transgenic and mutant plants, lateral root growth was significantly accelerated in the 35S::8ΔC transgenic plants but discernibly reduced in the ntl8-1 mutant. It is well known that lateral root growth is greatly affected by abiotic stresses, such as high salinity and drought.9 The NTL8 expression is markedly influenced by salt. The previous and our observations suggest that NTL8 may also be involved in root development in response to salt stress.
One distinct phenotype of the 35S::8ΔC transgenic plants is late flowering. Plants decide very carefully when to initiate flowering because it is essential for reproductive propagation. Therefore, plants constantly monitor internal and external environment, such as daylength, temperature, salinity, moisture, and so on.10–12 Since the 35S::8ΔC transgenic plants exhibit delayed flowering we examined the expression patterns of various flowering time genes in the transgenic plants. The expression of FT and of genes that act later in the flowering process, such as AP1, CAL, FUL and LEY, was greatly reduced in the transgenic plants, indicating that NTL8 regulates flowering initiation by modulating FT expression.
The expression of NTL8 is significantly induced by high salt, and the 35S::8ΔC transgenic plants is late flowering due to the repression of FT. Furthermore, it is known that high salt delays flowering in Arabidopsis, although the underlying molecular mechanisms are unknown.13 Therefore, an interesting question was whether FT is influenced by high salt or not.
A previous study has shown that FT is not affected by salt stress.13 However, it was still possible that daily rhythm or amplitude of the FT expression would be altered in the presence of high salt. To examine this possibility, wild type plants were treated with 100 mM NaCl and FT expression was measured during the time course of 24 hours under long day condition. Surprisingly, the FT transcript level was significantly reduced in plants treated with NaCl, especially during the period of 12–20 hours after dawn, when the FT transcript level is most high when grown under normal conditions.14 These results strongly support that high salt delays flowering by repressing FT. This view is also consistent with the salt responsiveness of NTL8 and the delayed flowering of the 35S::8ΔC transgenic plants in which FT is markedly repressed. Altogether, our observations suggest that controlled processing of the membrane-associated NTL8 transcription factor is important for its function and mediates salt stress responses in flowering time control via FT in Arabidopsis.
Although the expression of NTL8 is induced by high salt, there is no experimental evidence for the effects of high salt on the NTL8 protein processing. One possibility is that molecular components (or certain proteases) mediating NTL8 protein processing would exist only during specific developmental stages or in specific plant tissues. Actually, induction of NTL8 by high salt was higher in the roots rather than in the aerial plant parts. In addition, when a NTL8 promoter-GUS fusion was examined in transgenic plants, the GUS activity was primarily distributed in the vascular tissues of inflorescence stems and roots. It is well known that membrane-associated transcription factors are activated either by RUP or RIP.1 Our result showed that the stability of 8F and 8ΔC proteins was controlled by the ubiquitin/proteasome-mediated processing, but NTL8 protein processing might be regulated by unidentified intramembrane protease(s), like NTM1. We are currently under way to identify NTL8 processing protease(s).
Another interesting observation is that NTL8 may regulate salt stress signaling during seed germination. We recently found that the level of NTL8 transcript is extremely high in imbibed seeds. Seed germination is delayed in the presence of high salt. However, the ntl8-1 seeds were insensitive to high salt (unpublished). It will be interesting to investigate how the two developmental processes, flowering initiation and seed germination, are interrelated. It is possible that NTL8 may mediate the signaling crosstalks between these two processes (Fig. 1).
Figure 1.
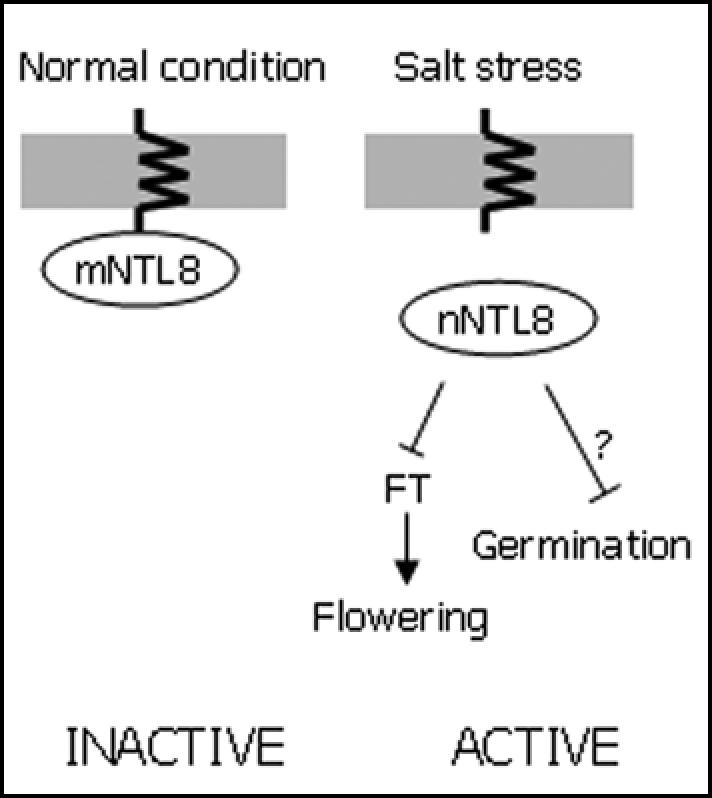
A proposed working scheme for NTL8 function in flowering time control and seed germination. NTL8 is liberated from the membranes triggered by an unidentified salt-induced molecular mechanism and enters the nucleus. In the nucleus, it represses FT expression, resulting in late flowering. NTL8 may also mediate seed germination through a molecular signaling.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4645
References
- 1.Hoppe T, Rape M, Jentsch S. Membrane-bound transcription factors: Regulated release by RIP or RUP. Curr Opin Cell Biol. 2001;13:344–348. doi: 10.1016/s0955-0674(00)00218-0. [DOI] [PubMed] [Google Scholar]
- 2.Kaffman A, O'Shea EK. Regulation of nuclear localization: A key to a door. Annu Rev Cell Dev Biol. 1999;15:291–339. doi: 10.1146/annurev.cellbio.15.1.291. [DOI] [PubMed] [Google Scholar]
- 3.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 4.Vik Å, Rine J. Membrane biology: Membrane-regulated transcription. Curr Biol. 2000;10:R869–R871. doi: 10.1016/s0960-9822(00)00822-8. [DOI] [PubMed] [Google Scholar]
- 5.Schwacke R, Schneider A, van der Graaff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flügge UI, Kunze R. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol. 2003;131:16–26. doi: 10.1104/pp.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SY, Kim SG, Kim YS, Seo PJ, Bae M, Yoon HK, Park CM. Exploring membrane-associated NAC transcription factors in Arabidopsis: Implications for membrane biology in genome regulation. Nucleic Acids Res. 2007;35:203–213. doi: 10.1093/nar/gkl1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YS, Kim SG, Park JE, Park HY, Lim MH, Chua NH, Park CM. A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell. 2006;18:3132–3144. doi: 10.1105/tpc.106.043018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SG, Kim SY, Park CM. A membrane-associated NAC transcription factor regulates salt-responsive Flowering via FLOWERING LOCUS T in Arabidopsis. Planta. 2007;226:647–654. doi: 10.1007/s00425-007-0513-3. [DOI] [PubMed] [Google Scholar]
- 9.Hegedus D, Yu M, Baldwin D, Gruber M, Sharpe A, Parkin I, Whitwill S, Lydiate D. Molecular characterization of Brassica napus NAC domain transcriptional activators induced in response to biotic and abiotic stress. Plant Mol Biol. 2003;53:383–397. doi: 10.1023/b:plan.0000006944.61384.11. [DOI] [PubMed] [Google Scholar]
- 10.Mouradov A, Cremer F, Coupland G. Control of flowering time: Interacting pathways as a basis for diversity. Plant Cell. 2000;14:S111–S130. doi: 10.1105/tpc.001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amasino RM. Vernalization and flowering time. Curr Opin Biotechnol. 2005;16:154–158. doi: 10.1016/j.copbio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Ausin I, Alonso-Blanco C, Martinez-Zapater JM. Environmental regulation of flowering. Int J Dev Biol. 2005;49:689–705. doi: 10.1387/ijdb.052022ia. [DOI] [PubMed] [Google Scholar]
- 13.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 14.Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]


