Abstract
The presence of the cyclic nucleotides 3′,5′-cyclic adenyl monophosphate (cAMP) and 3′,5′-cyclic guanyl monophosphate (cGMP) in plants is now generally accepted. In addition, cAMP and cGMP have been implicated in the regulation of important plant processes such as stomatal functioning, monovalent and divalent cation fluxes, chloroplast development, gibberellic acid signalling, pathogen response and gene transcription. However, very little is known regarding the components of cyclic nucleotide signalling in plants. In this addendum, the evidence for specific mechanisms of plant cyclic nucleotide signalling is evaluated and discussed.
Key Words: cAMP, cGMP, cyclic nucleotide, signalling, kinase
Introduction
All organisms have to transduce and decode information. Intracellular signalling molecules often function as intermediates in such transductions and these ‘second messengers’ include Ca2+, lipid based compounds and nucleotides such as 3′,5′-cyclic adenyl monophosphate (cAMP) and 3′,5′-cyclic guanyl monophosphate (cGMP). In animal cells the roles of the cyclic nucleotides cAMP and cGMP are well established and the basic mechanisms of cyclic nucleotide signalling and biochemistry have been well characterized (Fig. 1). In plants, the occurrence and roles of cAMP and cGMP have been more contentious. Nevertheless, sophisticated mass spectrometry-based analytical techniques unequivocally showed the presence of both cAMP and cGMP in several plant species.1 Several gene products with either adenylate2 or guanylate3 cyclase activity have been identified as has phosphodiesterase activity but strong functional evidence is still lacking in both cases.
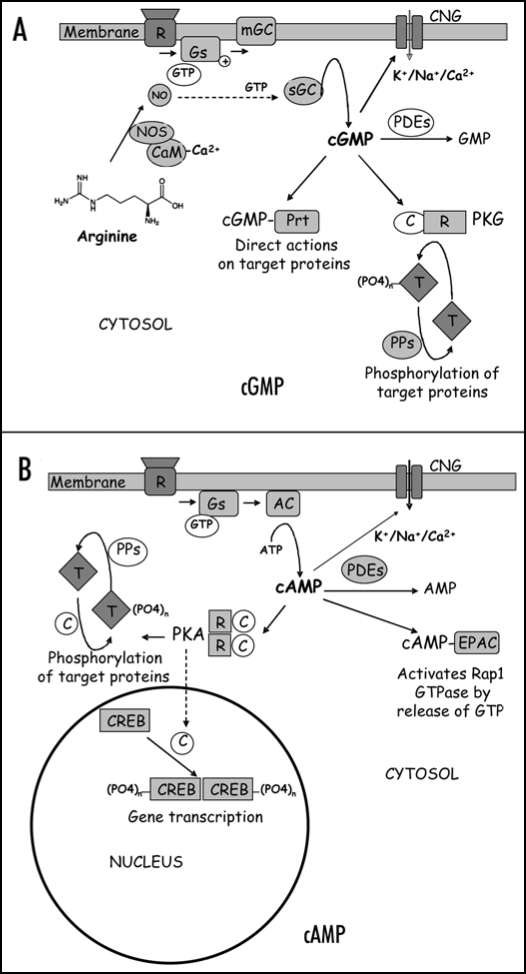
Figure 1.
Generic cyclic nucleotide transduction pathways in mammalian cells. (A) Many stimuli activate membrane bound (mGC) or soluble (sGC) guanylate cyclases, often in a nitric oxide (NO) and G-protein dependent manner, which leads to increased levels of cellular cGMP. Cyclic GMP is either broken down by specific phosphodiesterases (PDE) or can directly activates proteins such as cyclic nucleotide gated (CNG) channels. Alternatively, cGMP can activate cGMP-dependent kinase (PKG) which can phosphorylate a large number of target proteins (T). (B) Receptors (R) and G-proteins (Gs) are also involved in stimulation of adenylate cyclases (AC) generating an increase in cellular cAMP. Like cGMP, cAMP can directly modify the activity of proteins such as CNG channels, EPACs which activate Rap-GTPases and the cAMP dependent kinase PKA. Gene transcription is modulated via cAMP responsive element binding proteins (CREBs), transcription factors that dimerise upon phosphorylation by PKA. ‘NOS,’ NO synthase; ‘CaM,’ calmodulin; ‘PPs,’ phosphatases.
In spite of the seeming lack of clearly identifiable genes and proteins that mediate the basic biochemistry of cyclic nucleotides, many pharmacological and physiological studies implicate cAMP and cGMP in the regulation of important plant processes (Fig. 2). Reports from several laboratories suggest a role for cAMP and/or cGMP in stomatal opening.1 Studies using plant protoplasts4 and intact tissue5–8 have shown direct effects of cyclic nucleotides on cation fluxes sparking interest in these second messengers as potential regulators of the homeostasis of K+, Ca2+ and Na+. In agreement with this notion, recent work showed exposure of intact plants to NaCl generates an increase in cellular cGMP within seconds.9 In chloroplast development, cGMP has been demonstrated to be involved in the phytochrome mediated induction of the gene encoding chalcone synthase, a key enzyme in the biosynthesis of anthocyanins.10 Exposure of barley aleurone layers to gibberellic acid generates a transient increase in cGMP levels and gene transcription, which leads ultimately to α-amylase production for the conversion of starch into sugars.11 Transcription of defence genes such as PAL and PR-1 has been shown in response to nitric oxide (NO) with cGMP as an intermediate.12 Treatment of plants with membrane permeable cGMP affects the transcription of many genes, particularly of those encoding transporter proteins.8
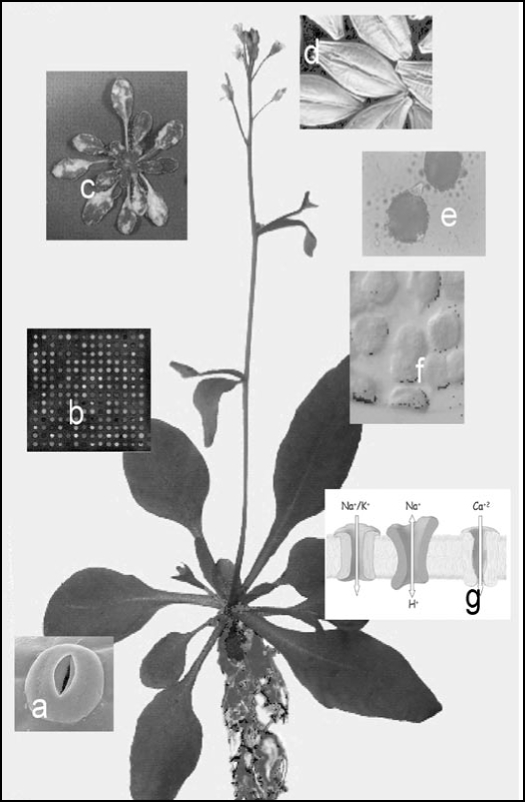
Figure 2.
Cyclic nucleotides have been implicated in many important plant processes such as stomatal functioning (a), gene transcription (b), pathogen attack (c), seed germination (d), pollen tube growth (e), chloroplast development (f) and cation fluxes (g).
A Role for Cyclic Nucleotide Dependent Kinases?
Clearly, cyclic nucleotide signalling has been shown to occur in plants and to be important for many cellular processes. In some cases, such as the modulation of cation fluxes, the effects can be explained through direct interaction between cyclic nucleotides and membrane transporters but in others, such as the modulation of gene transcription,8,11,12 the action of intermediate signalling components is almost inevitable. This raises the question what the components are in these cAMP and cGMP dependent signal transductions. In mammalian cells the activity of some proteins is directly modulated by cyclic nucleotides, (notably that of cyclic nucleotide gated channels or CNGCs), but the majority of cyclic nucleotide signal relays involves phosphorylation as an early down stream step (Fig. 1). The latter occurs through the action of cAMP (PKA) and cGMP (PKG) dependent kinases. Consequently, cyclic nucleotide dependent transcriptional modulation in animals is mediated by phosphorylation of cGMP and cAMP responsive transcription factors such as CREBs which affect transcription of hundreds of genes.13 PKA isoforms are also found in many fungal species.
In plants, the broad mechanisms of cyclic nucleotide signalling are still a mystery. A prime concern is the presence (or absence) of kinases that respond to changes in cellular cyclic nucleotide level. Conflicting reports are present whether cyclic nucleotide dependent kinase activity occurs in plants with some studies showing moderate rates of stimulation14 whereas others could not find any evidence.15 Bioinformatics analyses show the presence of unique genes in several plant genomes which contain both a cyclic nucleotide binding domain (CNBD) and a kinase domain16 (Fig. 3). In Arabidopsis, this putative cyclic nucleotide dependent kinase, At2g20040, shows a high degree of homology (E: 2.5 e−53) to mammalian type II PKG with around 48% similarity. While CNBD B of the supposed regulatory subunit appears rather degenerate, CNBD A is extremely well conserved, with only one invariant amino acid lacking. Problems are posed however by the absence of the canonical glycine rich loop (G50XG52XXG55) in domain I of the kinase moiety which is considered essential for nucleotide binding and of the catalytic aspartate in the YRD164–166 motif of domain VI. Such kinases are often considered ‘catalytically dead’ and in these cases the kinase domain is often suspected to deliver substrate/interactor binding specificity for other catalytic functions on the protein. Interestingly, the upstream part of what is the same open reading frame also contains a seemingly functional phosphatase domain, an arrangement which is unprecedented so far. The protein has several matching ESTs and promoter-GUS studies indicate it is expressed in vascular tissue and in developing shoot and flower tissues with an expression pattern closely corresponding to the zone of mitotic activity. Transcripts from an ortholog in Nicotiana tabacum BY-2 cells accumulate during the cell cycle in a pattern mirroring the fluctuations in cAMP content as described previously (ref. 17) (Roef L, personal communication). Preliminary data also suggest an antagonistic effect between ABA (inducing) and JA (inhibiting) on promoter activity of this gene (Van Ingelgem, Van Onckelen & Roef, personal communication). Unfortunately, expression of several transcript variants in E. coli either yields insoluble or inactive proteins. Whether this gene encodes a genuine cyclic nucleotide dependent kinase and/or phosphatase therefore asks for further scrutiny. No obvious phenotypes were observed in mutants with a disrupted kinase moiety in Arabidopsis (data not shown) but preliminary results from null mutations in the PP2C and CNBD domains hint at a physiological role during seed germination.
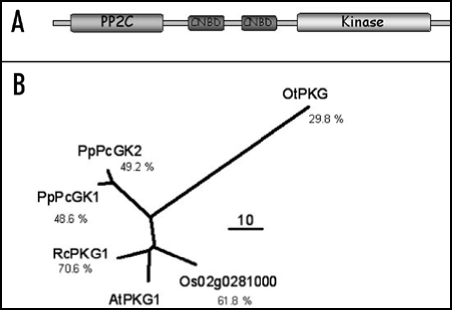
Figure 3.
(A) Conserved domain structure of a putative cyclic nucleotide dependent plant kinase. So far, it has been impossible to assign genuine kinase activity to this protein. Interestingly, a PP2C phosphatase moiety is also present in the same open reading frame but its functionality and significance are yet unknown. (B) A single copy gene is present in Arabidopsis thaliana, whereas homologous genes are found in the dicot Ricinus comunis, in the monocot Oryza sativa, in the moss Physcomitrella patens, and in the green alga Ostreococcus tauri. ‘PP2C’: phosphatase 2C; ‘CNBD’: cyclic nucleotide binding domain.
Alternative Mechanisms for Plant Cyclic Nucleotide Signalling
The evidence is so far stacked against the presence of either PKA or PKG in plants. However, such enzymes may have very low homology to their fungal and animal counterparts and therefore fail to be identified in bioinformatics analyses. Furthermore, their substrate specificity may also be divergent from that of kinases from other kingdoms, hampering the interpretation of biochemical assays which are typically carried out using ‘mammalian’ substrates such as glasstide and kemptide.15 Nevertheless, if no such kinases exist in plants, then how do cAMP and cGMP modulate their downstream targets? In plants, the largest group of proteins that contain CNBDs are the CNGCs. Although functional characterisation of plant CNGCs has been fraught with difficulties, there is now good evidence that (a) they are activated by binding cAMP and/or cGMP (b) they have well defined roles in pathogenesis and cation fluxes and (c) they can conduct Ca2+. The latter suggests that CNGCs may therefore provide a mechanism to convert cyclic nucleotide signals into Ca2+ signals, thus bypassing the need for cyclic nucleotide dependent kinases. The mechanisms of Ca2+ signalling in plants are fairly well established and include calcium-dependent protein kinases (CDPKs) and CIPKs (CBL-interacting protein kinases) which form a predominant group of Ca2+ sensors and effectors.18 CDPKs and CIPKs have been identified throughout the plant kingdom but do not occur in animals. Interestingly, CIPK structure shows strong resemblance to that of mammalian PKAs. A scenario where an initial cyclic nucleotide signal is converted into a Ca2+ signal could explain the well documented role of plant CNGCs in pathogen responses, the observation that Ca2+ fluxes occur during the early phase of plant defence responses, and the fact that both cAMP and cGMP have been implicated in plant defence-related signaling.12,19
In animals, EPACs (Exchange Protein directly Activated by cAMP) carry out diverse functions.20,21 EPACs contain GEFs (guanine nucleotide-exchange factors) that activate Rap GTPases upon binding to cAMP. However, EPAC orthologs have as yet not been identified in any plant genome.
Another family of candidate cyclic nucleotide targets consists of proteins carrying an amino terminal CNBD and a carboxyterminal acyl-CoA thioesterase domain. Several of these can be identified in plant genomes and such proteins would provide a means for cyclic nucleotides to impinge on fatty acid metabolism and possibly protein acylation processes. However, conservation of CNBD signatures is rather low in these proteins and whereas their thioesterase activity has been documented, neither cyclic nucleotide binding nor regulation by cyclic nucleotides has been demonstrated.22
In bacteria, cAMP is a cofactor of the cAMP-receptor protein (CRP) rather than an activator of protein kinases.23 CRP or Catabolite gene Activator Protein (CAP) directly binds to DNA upstream of promoters and can either repress or activate gene transcription. CRP/CAP like proteins show some similarity to plant shaker type channels but are otherwise not apparent in plant genomes. The GAF domain (found in cGMP-specific phosphodiesterases, Adenylyl cyclases and Formate hydrogen lyases) is another domain that binds cyclic nucleotides but probably evolved independently of the CNBD.15 GAFs are particularly prevalent in bacteria23 where they are believed to allosterically regulate catalytic activities of for example adenylate cyclases, after binding cGMP. GAF domains are also prevalent in green plants, and are found especially in phytochromes and (putative) ethylene receptors. Such direct modulation of phytochromes via their GAF domain would provide a neat explanation of the role of cGMP in chloroplast development.
Conclusions and Outlook
Evidence for the presence and physiological relevance of cAMP and cGMP based signalling in plants is now overwhelming. Yet, some of the key components of these transductions need urgent clarification. Evidence for bona fide PKA and PKG type kinases is scarce but cannot be completely dismissed. Suggestions that the phosphatase function is the key activity of several candidate cyclic nucleotide dependent phosphatase/kinases, may open new perspectives on cyclic nucleotide dependent phosphorylation events in plants. Alternative mechanisms could rely on the conversion of cyclic nucleotide signals into Ca2+ signals via CNGCs or on more specific and direct modulation of protein activity via GAF domains on phytochromes. The development of non invasive fluorescent reporters24 for the real time recording of cyclic nucleotides in plant cells (2) would be a great help to further delineate the specific roles of cAMP and cGMP but the main requirement is an unequivocal identification and functional characterisation of (signalling) proteins that bind cAMP and cGMP.
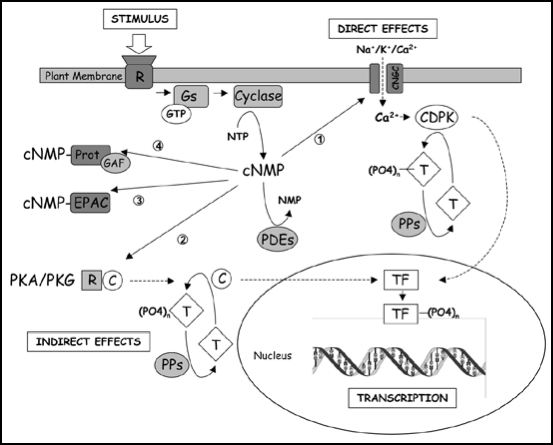
Figure 4.
Possible cyclic nucleotide transduction pathways in plants. (1) Cyclic nucleotide gated channels have been characterised in plants and are known to play important roles in pathogen response and cation transport. In addition, they can form a node in the conversion of cyclic nucleotide signals into Ca2+ based signalling which can be processed through Ca2+ sensors such as Ca2+ dependent kinases(CDPKs) and the Ca+2 signaling components CIPK-CBL. (2) Although most evidence points to its absence, it cannot be ruled out that, in analogy to mammalian cells, plants contain specific kinases that are activated by cyclic nucleotides. (3) EPACs can activate Rap-GTPases but none has been identified in plants so far. (4) Many plant proteins contain GAF domains but whether these bind and are modulated by cyclic nucleotides remains to be determined.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4789
References
- 1.Newton RP, Smith CJ. Cyclic nucleotides. Phytochemistry. 2004;65:2423–2437. doi: 10.1016/j.phytochem.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 2.Moutinho A, Hussey PJ, Trewavas AJ, Malho R. cAMP acts as a second messenger in pollen tube growth and reorientation. Proc Natl Acad Sci USA. 2001;98:10481–10486. doi: 10.1073/pnas.171104598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludidi N, Gehring C. Identification of a novel protein with guanylyl cyclase activity in Arabidopsis thaliana. J Biol Chem. 2003;278:6490–6494. doi: 10.1074/jbc.M210983200. [DOI] [PubMed] [Google Scholar]
- 4.Volotovski ID, Sokolovski SG, Molchan OV, Knight MR. Second messengers mediate increases in cytosolic calcium in tobacco protoplasts. Plant Physiol. 1998;117:1023–1030. doi: 10.1104/pp.117.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maathuis FJM, Sanders D. Sodium uptake in Arabidopsis thaliana roots is regulated by cyclic nucleotides. Plant Physiol. 2001;127:1617–1625. [PMC free article] [PubMed] [Google Scholar]
- 6.Essah PA, Davenport R, Tester M. Sodium influx and accumulation in Arabidopsis. Plant Physiol. 2003;133:307–318. doi: 10.1104/pp.103.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio F, Flores P, Navarro JM, Martinez V. Effects of Ca2+, K+ and cGMP on Na+ uptake in pepper plants. Plant Sci. 2003;165:1043–1049. [Google Scholar]
- 8.Maathuis FJM. cGMP modulates gene transcription and cation transport in Arabidopsis roots. Plant J. 2006;45:700–711. doi: 10.1111/j.1365-313X.2005.02616.x. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson L, Ludidi N, Knight MR, Gehring C, Denby K. Salt and osmotic stress cause rapid increases in Arabidopsis thaliana cGMP levels. FEBS Letts. 2004;569:317–320. doi: 10.1016/j.febslet.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Bowler C, Neuhaus G, Yamagata H, Chua NH. Cyclic GMP and calcium mediate phytochrome phototransduction. Cell. 1994;77:73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 11.Penson SP, Schuurink RC, Fath A, Gubler F, Jacobsen JV, Jones RL. cGMP is required for gibberellic acid-induced gene expression in barley aleurone. Plant Cell. 1996;8:2325–2333. doi: 10.1105/tpc.8.12.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey J, Tyson-Capper AJ, Gilmore K, Robson SC, Europe-Finner GN. Identification of human myometrial target genes of the cAMP pathway: The role of cAMP-response element binding (CREB) and modulator CREM alpha and CREM tau2alpha proteins. J Mol Endocrin. 2005;34:1–17. doi: 10.1677/jme.1.01594. [DOI] [PubMed] [Google Scholar]
- 14.Szmidt-Jaworska A, Jaworski K, Tretyn A, Kopcewicz J. Biochemical evidence for a cGMP-regulated protein kinase in Pharbitis nil. Phytochemistry. 2003;63:635–642. doi: 10.1016/s0031-9422(03)00247-4. [DOI] [PubMed] [Google Scholar]
- 15.Bridges D, Fraser ME, Moorhead GBG. Cyclic nucleotide binding proteins in the Arabidopsis thaliana and Oryza sativa genomes. Bmc Bioinformatics. 2005;6:1–12. doi: 10.1186/1471-2105-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krupa A, Anamika, Srinivasa N. Genome-wide comparative analyses of domain organisation of repertoires of protein kinases of Arabidopsis thaliana and Oryza sativa. Gene. 2006;380:1–13. doi: 10.1016/j.gene.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Ehsan H, Reichheld JP, Roef L, Witters E, Lardon F, Van Bockstaele D, Van Montagu M, Inzé D, Van Onckelen H. Effect of indomethacin on cell cycle dependent cyclic AMP fluxes in tobacco BY-2 cells. FEBS Letts. 1998;422:165–169. doi: 10.1016/s0014-5793(97)01610-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Lu YT. Calmodulin-binding protein kinases in plants. Trends Plant Sci. 2003;8:123–127. doi: 10.1016/S1360-1385(03)00013-X. [DOI] [PubMed] [Google Scholar]
- 19.Clough SJ, Fengler KA, Yu I, Lippok B, Smith RK, Bent A. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA. 2000;97:9323–9328. doi: 10.1073/pnas.150005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 22.Tilton GB, Shockey JM, Browse J. Biochemical and molecular characterization of ACH2, an acyl-CoA thioesterase from Arabidopsis thaliana. J Biol Chem. 2004;279:7487–7494. doi: 10.1074/jbc.M309532200. [DOI] [PubMed] [Google Scholar]
- 23.Cann MJ. Signalling through cyclic nucleotide monophosphates in cyanobacteria. New Phytol. 2004;161:1–23. [Google Scholar]
- 24.Nikolaev VO, Lohse MJ. Monitoring of cAMP synthesis and degradation in living cells. Physiology. 2006;21:86–92. doi: 10.1152/physiol.00057.2005. [DOI] [PubMed] [Google Scholar]