Abstract
Programmed cell death (PCD) is an integrated cellular process occurring in plant growth, development, and defense responses to facilitate normal growth and development and better survival against various stresses as a whole. As universal toxic chemicals in plant and animal cells, reactive oxygen or nitrogen species (ROS or RNS), mainly superoxide anion (O2−•), hydrogen peroxide (H2O2) or nitric oxide (•NO), have been studied extensively for their roles in PCD induction. Physiological and genetic studies have convincingly shown their essential roles. However, the details and mechanisms by which ROS and •NO interplay and induce PCD are not well understood. Our recent study on Cupressus lusitanica culture cell death revealed the elicitor-induced co-accumulation of ROS and •NO and interactions between •NO and H2O2 or O2-• in different ways to regulate cell death. •NO and H2O2 reciprocally enhanced the production of each other whereas •NO and O2−• showed reciprocal suppression on each other's production. It was the interaction between •NO and O2-• but not between •NO and H2O2 that induced PCD, probably through peroxynitrite (ONOO−). In this addendum, some unsolved issues in the study were discussed based on recent studies on the complex network of ROS and •NO leading to PCD in animals and plants.
Key Words: cell death, nitric oxide, reactive oxygen species, interaction, posttranslational modification
Introduction
Biosynthesis of µ-thujaplicin is of great interest not only because of its novel structure but also for its multiple biological activities and an increasing demand in market.1 Fungal elicitor -induced µ-thujaplicin production by Cupressus lusitanica cell culture results from several signaling mechanisms, while the elicitor-induced hypersensitive cell death was mediated in part by elicitor-induced O2−• but not H2O2.2,3 It was found that •NO was generated upon elicitor treatment in parallel with O2−• and H2O2 accumulation, and •NO donors also induced a pronounced C. lusitanica cell death. Using biosynthetic enzyme inhibitors or scavengers, we showed that •NO and O2−• production was necessary for O2−• or •NO induction of cell death, respectively. Measuring H2O2, •NO and O2−• production in various treatments indicated that H2O2 and •NO reciprocally stimulated the production of each other, whereas •NO and O2−• suppressed the accumulation of each other. Since •NO readily reacts with O2−• and generate a more potent oxidant ONOO−, which plays pivotal roles in animal cells under oxidative and nitrosative stress, we proposed that •NO and O2− induced cell death mainly through their interaction product ONOO−.
ROS-or RNS-Induced Cell Death is More Likely Mediated by Interaction between ROS and RNS
Early physiological studies have shown many controversial results about if ROS and •NO, either O2−• or H2O2 or •NO, is necessary or sufficient to induce plant cell death.4–7 Genetic evidence has proved that elevated O2−, singlet oxygen, or H2O2 levels are able to induce cell death under certain conditions. Arabidopsis mutants lsd1 and rcd1 produce more O2− and thus undergo a PCD spontaneously.8,9 A flu mutant generates singlelet oxygen (•O2) upon a dark-to-light shift and initiates a cell death.10 Transgenic plants deficient in H2O2-scavenging enzymes such as ascorbate peroxidase (APX) and catalase have elevated ROS levels, and therefore are more susceptible to •NO treatment by showing a more dramatically augmented cell death than wild-type plants.11,12 These data suggest that elevated ROS levels in these plants are necessary for cell death induction. However, it may be too early to conclude that these ROS alone are sufficient to induce PCD since whether •NO is involved in initiating cell death by ROS in these plants was not tested. On the other hand, it has been shown that •NO requires the well-balanced H2O2 levels to induce plant cell death.4,5 It is more likely that ROS and •NO interactions undergoing in these plants might be the real causes for cell death.
Many studies involved the use of exogenous •NO donors, high dose of H2O2 or O2−• generation systems. In fact, application of •NO donors to cells can induce ROS production,13,14 •NO donor-induced cell death thus can be attributable to interactions between •NO and ROS. Similarly, treatment with H2O2 or O2−• generation system may also evoke •NO generation.15,16 ROS-induced cell death thus may not be solely caused by H2O2 or O2−•, but involve •NO. The advanced studies on animal cells already convincingly demonstrate that NO-induced cell death largely attribute to its interaction with O2−• and the interaction product ONOO−, although the early study on animals showed that •NO hasten apoptosis in macrophages, thymocytes, and tumor cells.14 However, there is a few data available for O2−• interaction with •NO and ONOO− production and function in plant. The lack of convenient and accurate tools to precisely capture and measure ONOO− and other ROS-RNS interaction chain products may partly contribute to this situation. Several early experiments indicated the inhibition of O2−• on •NO production and vise versa.17,18 We clearly demonstrated such interaction in plant cells and their correlations with cell death. Yet some important questions remain to be solved: what's role of transit metal Fe2+ in culture medium for ROS and •NO interaction; how H2O2, O2− and •NO affect each other's accumulation at enzyme levels; how much ONOO− generated for cell death induction or anti-lipid peroxidation; what are downstream targets of ROS and •NO interactions and intermediates for cell death induction. All these issues are critical for understanding ROS and •NO signaling leading to PCD. Recent progress from animal and plant studies has provided many clues for addressing these questions.14,19
Posttranslational Modifications of Proteins by RNS and ROS are Important Mechanisms of Cell Death Induction
ROS and •NO are readily diffusible short-lived free radicals, thus can react with a variety of intracellular and extracellular targets, through which numerous physiological and cellular functions of them are achieved. •NO interacts with transition metals and this reaction can cause modification of many metalloproteins, who have metal binding sites at active center. •NO interacts with tobacco aconitase that catalyze the isomerisation of citrate to isocitrate.20 In animals, •NO interacted with guanylate cyclase (GC), which generates cGMP and induces Ca2+ fluctuation.21 Soluble GC directly binds to •NO at heme moiety of N-terminus, followed by conformational change and activation of enzyme. •NO binds to the oxidized form cytochrome c oxidase (Cyt C oxidase) at the binuclear heme-copper center as a competitive inhibitor of oxygen binding and inactivates the enzyme.21 •NO interacts with heme-containing enzymes catalase and peroxidases and inhibits their activities.13 This was also confirmed in C. lusitanica cell cultures.
Thiol modification by ROS such as hydrogen peroxide is already recognized as a potential signalling mechanism in plants.19 S-Nitrosylation of cysteine residues in proteins has been well documented in animals.22 S-nitrosylation of plant proteins is also regarded as an important regulatory mechanism similar to that of protein phosphoralytion. S-Nitrosylation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), hemoglobin and Met adenosyltransferase has been reported; more substrate proteins have been identified as potentially S-nitrosylated targets in Arabidopsis.23 H2O2 also modified and inhibited GAPDH.24
Tyrosine nitration is mediated by ONOO− and nitrogen dioxide (•NO2) formed as interaction between RNS and ROS or transition metals. In animals, peroxynitrite mediates the inactivation of Mn-superoxide dismutase (Mn-SOD) through tyrosine nitration at Tyr34 into 3-nitrotyrosine.25 This modification also activates cytochrome c.26 Nitration of Tyr residues in plant proteins has also been observed in antisense nitrite reductase tobacco27 and following administration of ONOO− in vitro.4 Tyrosine nitration causes conformation, structure or catalytic activity changes; it also could block protein phosphorylation, which may be one mechanism for activation of target proteins.
All •NO or ROS, or ONOO- mediated protein modifications can be essential cellular processes for PCD induction, not only by activation or inactivation of RNS/ROS generating and metabolizing enzymes but also through direct regulation of signaling and cellular process of PCD such as Ca2+ fluctuation, procaspase-3 activation.22
Balances of ROS and RNS may Control PCD or Necrotic Cell Death
ROS and •NO generate under both stress situations and normal growth conditions, thus ROS and •NO interaction and ONOO− may form universally.14,19 Animals and plants developed enzyme-and non-enzyme-based systems to scavenge excessive ROS and RNS to keep the balances between generation and depletion of ROS and RNS, such as Mn-SOD, catalase, APX and glutathione-based systems.5,14,15 S-Nitrosoglutathione (GSNO) from rapid reaction of •NO and glutathione, or methemoglobin, can be the ways to remove •NO or as reservoirs or donors of •NO.28 Peroxiredoxins are likely involved in reduction of both ROS and peroxynitrite.29 However, loss of these balances during pathogenesis or abiotic stresses results in oxidative and nitrosative stresses, where ROS and RNS are overproduced. A plenty of evidence suggest that balances of RNS (mainly •NO) and ROS production are important for directing physiological consequences, either better survival or cell death.4,15,30 C. lusitanica culture cells have strong capability to scavenge H2O2, just like other cell cultures, and thus physiological concentrations of H2O2 do not induce a significant cell death.2 High Fe2+ in culture medium induced cell death and strong lipid peroxidation through Fenton reaction, and also induced β-thujaplicin biosynthesis. However, glutathione enhanced both H2O2 and Fe2+-induced β-biosynthesis although it reduced lipid peroxidation, which was proposed to lead to oxylipin signaling for β-thujaplicin induction.2 This may suggest GSNO from •NO- glutathione played roles in β-thujaplicin biosynthesis.
•NO-induced H2O2 production or H2O2-induced •NO accumulation may not be only due to suppression of catalase and activation of NADPH oxidase or •NO-generating enzymes, other reasons such as ROS- and RNS- mediated radical self-propagation mechanisms also can contribute to the results. Particularly in C. lusitanica cells, interactions between NO and ROS and their interactions with Fe2+ or other free radicals, such as lipid radicals, may formed much more complicated chain reactions beyond our current understanding.3,31,32 RNS, ROS and lipid free radicals often form self-propagated chains and generate a large number of toxic oxidants, which can cause various cellular effects from modulations of cell signaling to overwhelming oxidative injury on lipids, DNA and proteins as well as integrity of both plasma- or endo-membranes, and eventually commit cells to necrosis or PCD.14 On enzyme levels, except affecting catalase and APX activity, •NO binding to Cyt C oxidase reversibly inhibits its activity and, as a consequence, increases O2−• and H2O2 production. 21 ONOO− mediated tryrosine nitration of Mn-SOD inhibit Mn-SOD activity, which results in over-accumulation of O2− • and ONOO−.21
On the other hand, some of the spontaneous and rapid chain reactions of RNS and ROS may scavenge some toxic radicals and protect cells from oxidative or nitrosative damaging.31 Guo and Crawford showed that deficiency in •NO production deficiency in AtNOS1 mutant resulted in unbalanced accumulation of ROS.34 In our study, higher •NO production obviously inhibited lipid peroxidation. This often observed concentration-dependent function changes may reflect the importance of balance between RNS and ROS and their interactions.4,30 The different fates resulted from these reactive free radicals also depend on physiological environments that plant cells are in. Details and mechanisms for these aspects remain to be elusive.
ROS-RNS Induced Cell Death Initiates from Dysfunction of Mitochondria
Unlike necrotic cell death caused by excessive phytotoxics, PCD is a cell death and involves many reversible molecular processes and cellular machineries. The cellular components for both ROS and •NO signaling pathways leading to cell death include Ca2+ spiking, Ca2+-binding proteins, protein kinases such as MAPKs, caspase or caspase-like proteases, lipid messengers such as phosphatidic acid and fatty acid hydroperoxides.19,32,33 The apoptosis and necrosis in animals all are originated from the mitochondria, where ROS and •NO can be excessively generated during pathogenesis.35 The mitochondrial dysfunction such as permeability transition, cytochrome c release and respiration inhibition caused by ROS and RNS stresses, as well as their interaction results.36,37 Cytochrome c release is necessary for caspase activation that precedes mitochondrial permeability transition, nuclear condensation, and other hallmarks of apoptosis in animals.35 Mitochondria also serve as an essential place for launching plant cell death because ROS and •NO stresses are amplified in mitochondria to trigger cytochrome c release through mitochondrial transition pore opening and morphological changes.36,37 One of the interesting observations for elicitor-induced PCD in C. lusitanica cell culture is treachery element differentiation. This xylogenesis PCD may involve •NO and ROS signaling as reported by others.38 Further study is required to illustrate this issue.
In conclusion, ROS and •NO coincidently occur within the same subcellular organelles, such as the mitochondria, in response to biotic and abiotic stresses, and their levels can be reciprocally controlled or affected by each other through the direct modification of enzymes involved in synthesis or catabolism of •NO and ROS.39 •NO and ROS often show some overlapping and synergistic functions, particularly in cell death induction through interactions, which are determined by their reactive natures. The production balances of and diverse interactions between ROS and •NO under different physiological environments form a complex signaling cellular network to determine if plant cells continue to survive or are directed to death. These redox signaling and complex cellular processes play essential roles in innate immune response and other defense systems of plants.
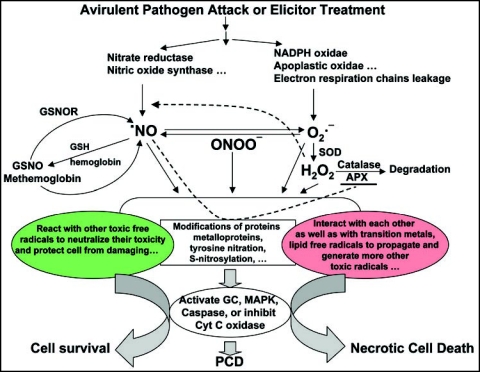
Figure 1.
Schematic of reactive nitrogen and oxygen species interaction network in pathogen- or elicitor-induced cell death. Avirulent pathogen attack or elicitor treatment induces signal transduction leading to reactive oxygen species (ROS), O2−• and H2O2, production, mainly by NADPH oxidase or apolplastic peroxidase, and NO production mainly by membrane-bond nitrate reductase or nitric oxide synthase (NOS). O2−• is also generated in the mitochondria through electron respiration chain leakage and •NO is generated there by AtNOS1. •NO can be released from S-nitrosoglutathione (GSNO) by S-nitrosoglutathione reductase (GSNOR) or methemoglobin through reversible reaction with glutathione (GSH) or hemoglobin. •NO and O2− rapidly react and produce peroxynitrite (ONOO−), O2−• is dismuted spontaneously or by SOD into H2O2. •NO, ONOO−, O2−• and H2O2 or their interaction products such as S-nitrosoglutathione (GSNO)- mediated posttranslational modifications of proteins (reaction with metalloproteins, tyrosine nitraton or S-nitrosylation) play essential roles in •NO- and ROS-induced cell death. The modifications may inhibit cytochrome C oxidase (Cyt C oxidase) or activate guanylate cyclase (GC), mitogen-activated protein kinase (MAPK) and caspase or caspase-like proteases, and then activate mitochondria dysfunction pathway leading to PCD. While direct interactions of ROS and reactive nitrogen species (RNS) with each other as well as transition metals and lipid free radicals can form self-propagating chains, with the help of posttranslational modification by •NO or RNS of ROS-generating or catabolism enzymes such as APX, Mn-SOD, catalase and Cyt C oxidase. Such overproduction of various toxic free radicals can cause necrotic cell death. On the other hand, •NO- or RNS reaction products -mediated modifications may be in favor of antioxidant or anti-lipid peroxidation properties of •NO or RNS reaction products, which can neutralize their toxicities of these reactive free radicals and thereby protect cells from damaging. Under such situations, most cells will survive in cost of some cells undergoing PCD. Balances of ROS and RNS production, radical interactions and protein modifications may determine the fates of involving cells and regulate innate immune defense responses of plants.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4802
References
- 1.Zhao J. Plant troponoids: Chemistry, biological activity, and biosynthesis. Curr Med Chem. 2007;14:2597–2621. doi: 10.2174/092986707782023253. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Fujita K, Sakai K. Oxidative stress in plant cell culture: A role in production of β-thujaplicin by Cupressus lusitanica cell cultures. Biotechnol Bioeng. 2005;90:621–631. doi: 10.1002/bit.20465. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Davis L, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Pinto MC, Tommasi F, De Gara L. Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright-Yellow 2 cells. Plant Physiol. 2002;130:698–708. doi: 10.1104/pp.005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL. Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 2002;129:1642–1650. doi: 10.1104/pp.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tada Y, Mori T, Shinogi T, Yao N, Takahashi S, et al. Nitric oxide and reactive oxygen species do not elicit hypersensitive cell death but induce apoptosis in the adjacent cells during the defense response of oat. Mol Plant-Microbe Interact. 2004;17:245–253. doi: 10.1094/MPMI.2004.17.3.245. [DOI] [PubMed] [Google Scholar]
- 8.Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in a Arabidopsis mutant by extracellular superoxide. Science. 1996;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- 9.Overmyer K, Brosche M, Pellinen R, Kuittinen T, Tuominen H, Ahlfors R, Keinanen M, Saarma M, Scheel D, Kangasjarvi J. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death1 mutant. Plant Physiol. 2005;37:1092–1104. doi: 10.1104/pp.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Gobel C, Feussner I, Nater M, Apel K. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarantino D, Vannini C, Bracale M, Campa M, Soave C, Murgia I. Antisense reduction of thylakoidal ascorbate peroxidase in Arabidopsis enhances paraquat-induced photooxidative stress and nitric oxide-induced cell death. Planta. 2005;221:757–765. doi: 10.1007/s00425-005-1485-9. [DOI] [PubMed] [Google Scholar]
- 12.Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inzé D, Delledonne M, Van Breusegem F. Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol. 2006;141:404–411. doi: 10.1104/pp.106.078444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark D, Durner J, Navarre DA, Klessig DF. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant-Microbe Interact. 2000;13:1380–1384. doi: 10.1094/MPMI.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- 14.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Pinto MC, Paradiso A, Leonetti P, De Gara L. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J. 2006;48:784–795. doi: 10.1111/j.1365-313X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- 16.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 17.Caro A, Puntarulo S. Nitric oxide decreases superoxide anion generation by microsomes from soybean embryonic axes. Physiol Plantarum. 1998;104:357–364. [Google Scholar]
- 18.Vanin F, Svistunenko DA, Mikoyan VD, Serezhenkov VA, Fryer MJ, Baker NR, Cooper CE. Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. J Biol Chem. 2004;279:24100–24107. doi: 10.1074/jbc.M312601200. [DOI] [PubMed] [Google Scholar]
- 19.Zaninotto F, Camera SL, Polverari A, Delledonne M. Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiol. 141:379–383. doi: 10.1104/pp.106.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarre DA, Wendehenne D, Durner J, Noad R, Klessig DF. Nitric oxide modulates the activity of tobacco aconitase. Plant Physiol. 122:573–582. doi: 10.1104/pp.122.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:L262–L268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 22.Benhar M, Stamler JS. A central role for S-nitrosylation in apoptosis. Nature Cell Biol. 2005;7:645–646. doi: 10.1038/ncb0705-645. [DOI] [PubMed] [Google Scholar]
- 23.Lindermayr C, Saalbach G, Durner J. Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol. 137:921–930. doi: 10.1104/pp.104.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hancock JT, Henson D, Nyirenda M, Desikan R, Harrison J, Lewis M, Hughes J, Neill SJ. Proteomic identification of glyceraldehyde 3-phosphate dehydrogenase as an inhibitory target of hydrogen peroxide in Arabidopsis. Plant Physiol Biochem. 2005;4:828–835. doi: 10.1016/j.plaphy.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- 26.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 27.Morot-Gaudry-Talarmain Y, Rockel P, Moureaux T, Quillere I, Leydecker MT, Kaiser WM, Morot-Gaudry JF. Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense tobacco. Planta. 2002;215:708–715. doi: 10.1007/s00425-002-0816-3. [DOI] [PubMed] [Google Scholar]
- 28.Rusterucci C, Espunya MC, Diaz M, Chabannes M, Martinez MC. S-nitrosoglutathione reductase affords protection against pathogens in Arabidopsis, both locally and systemically. Plant Physiol. 2007;143:1282–1292. doi: 10.1104/pp.106.091686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 30.Espey MG, Miranda KM, Feelisch M, Fukuto J, Grisham MB, Vitek MP, Wink DA. Mechanisms of cell death governed by the balance between nitrosative and oxidative stress. Ann NY Acad Sci. 2000;899:209–221. doi: 10.1111/j.1749-6632.2000.tb06188.x. [DOI] [PubMed] [Google Scholar]
- 31.Rubbo H, Radi R, Anselmi D, Kirk M, Barnes S, Butler J, Eiserich JP, Freeman BA. Nitric oxide reaction with lipid peroxyl radicals spares a-tocopherol during lipid peroxidation. Greater oxidant protection from the pair nitric oxide/ α -tocopherol than α -tocopherol/ascorbate. J Biol Chem. 2000;275:10812–10818. doi: 10.1074/jbc.275.15.10812. [DOI] [PubMed] [Google Scholar]
- 32.Montillet JL, Chamnongpol S, Rustérucci C, Dat J, Van de Cotte B, Agnel JP, Battesti C, Inzé D, Van Breusegem F, Triantaphylidès C. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 2005;138:1516–1526. doi: 10.1104/pp.105.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laxalt AM, Raho N, Have AT, Lamattina L. Nitric oxide is critical for inducing phosphatidic acid accumulation in xylanase-elicited tomato cells. J Biol Chem. 2007;282:21160–21168. doi: 10.1074/jbc.M701212200. [DOI] [PubMed] [Google Scholar]
- 34.Guo FQ, Crawford NM. Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell. 2005;17:3436–3450. doi: 10.1105/tpc.105.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borutaite V, Jekabsone A, Morkuniene R, Brown GC. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J Mol Cell Cardiol. 2003;35:357–366. doi: 10.1016/s0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 36.Vacca RA, Valenti D, Bobba A, Merafina RS, Passarella S, Marra E. Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock-induced cell death. Plant Physiol. 2006;141:208–219. doi: 10.1104/pp.106.078683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morimoto S, Tanaka Y, Sasaki K, Tanaka H, Fukamizu T, Shoyama Y, Shoyama Y, Taura F. Identification and characterization of cannabinoids that induce cell death through mitochondrial permeability transition in Cannabis leaf cells. J Biol Chem. 2007;282:20739–20751. doi: 10.1074/jbc.M700133200. [DOI] [PubMed] [Google Scholar]
- 38.Gabaldón C, Gómez Ros LV, Pedreño MA, Barceló AR. Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytol. 2005;165:121–130. doi: 10.1111/j.1469-8137.2004.01230.x. [DOI] [PubMed] [Google Scholar]
- 39.Planchet E, Kaiser WM. Nitric oxide production in plants: Facts and fictions. Plant Signal Behav. 2006;1:46–51. doi: 10.4161/psb.1.2.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]