Abstract
In animals, sorting of membrane proteins following their internalization from the plasma membrane (PM) by endocytosis occurs through a series of different endosomal compartments. In plants, how and where these sorting events take place is still poorly understood and our current view of the endocytic pathway still largely relies on analogies made from the animal system. However, extensive differences seem to exist between animal and plant endosomal functions, as exemplified by the role of the trans-Golgi network (TGN) as an early endosomal compartment in plants or the functional diversification of conserved sorting complexes. By using the Arabidopsis root tip as a reference model, we and other have begun to shed light on the complexity of the plant endocytic pathways. Notably, we have recently characterized the functions of an endosomal compartment, the SNX1-endosomes, also referred to as the prevacuolar compartment (PVC) or multivesicular bodies (MVB), in the sorting of different cargo proteins, including two related auxin-efflux carriers, PIN1 and PIN2. We have shown that routing decisions take place at this endosomal level, such as the sorting of PIN2 toward the lytic vacuole for degradation or PIN1 toward the PM for recycling.
Key Words: Arabidopsis, intracellular trafficking, endocytic recycling, endosomes, MVB, PVC, VPS29, SNX, PIN, cell polarity
The Arabidopsis Root Tip as a Toolbox to Study Endocytosis in Plants
Endocytosis has been discovered in intact plant tissues only recently and is nowadays the center of much attention as a plethora of emerging functions are attributed to this cellular process, such as the regulation of cell polarity, cell signaling or cell cytokinesis.1–11 Our fast growing knowledge on plant endocytosis comes mainly from the recent availability of robust intracellular compartment markers,1,4,6–8,12,13 the discovery of cargo proteins4,5,9–11,13 and the use of trafficking inhibitors.1,5–7,10,13 A model of choice to study endocytosis in plants is the Arabidopsis root tip as it revealed to be particularly suitable for cell imaging and is readily accessible to drug and dye treatments. Indeed, the sensitivity of root cells to the trafficking inhibitor Brefeldin A (BFA) was instrumental in understanding plant endocytosis.5 In root cells, BFA primarily interferes with endosomal compartments,1,4–7,14 whereas in most of the green tissues, it targets the endoplasmic reticulum (ER)-to-Golgi traffic.12,14–16 Certain confusing results in the field of plant endocytosis came from the fact that the same drug (e.g., BFA) may trigger different effects depending on the cell type. For instance, Wortmannin (Wm) selectively targets MVBs in Arabidopsis root tips,6,7 but targets both MVB and TGN compartments in tobacco BY-2 cells.17,18 Another example is the FM4-64 labeling, which has been widely used as an endocytic tracer in plants.1,3,4,6–10,12,13,16,19–21 FM4-64 colocalizes with Golgi bodies after 30 min of internalization in BY-2 cells,20 whereas it colocalizes only faintly with Golgi compartments in the Arabidopsis root tip, even after longer exposure to the dye (our unpublished observations and refs. 12 and 13).
Due to these variations in drug sensitivity, we decided to focus only on the Arabidopsis root system and we produced a collection of Arabidopsis transgenic lines that express fluorescent-tagged markers of intracellular compartments.6,7 This effort, combined with those of other laboratories, enabled the establishment of stable, functional reporter lines for almost all the intracellular compartments in the endocytic or secretory pathways (for example see refs. 1,4,7,12 and Fig. 1A). This collection was used to analyze the sensitivity of each intracellular compartment to different dyes or drugs, such as FM4-64, the sterol dye filipin, BFA, Wm, Concanamycin A or Tyrphostin A23,1,6,10,13 in the same and unique root model system. Finally, an obvious advantage of the Arabidopsis system is to allow the combination of cellular with genetic or reverse genetic approaches.4,8,12,16,22
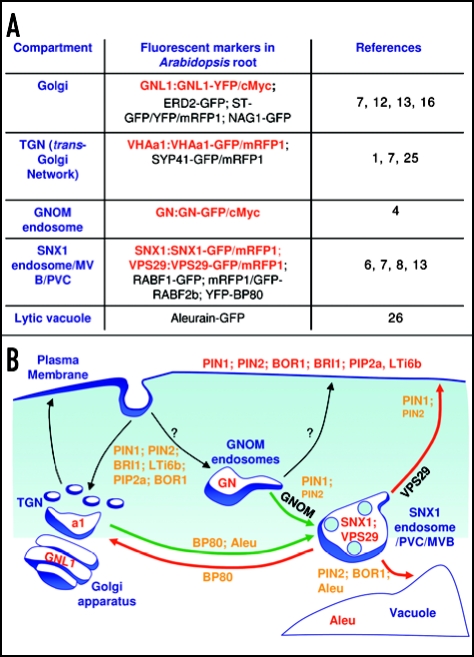
Figure 1.
Intracellular compartment markers and trafficking pathways in Arabidopsis root cells. (A) Table indicating stably transformed Arabidopsis lines expressing fluorescent intracellular compartment markers. In red, functional fusion proteins under the control of their own promoter. (B) Schematic representation of the endocytic pathways in Arabidopsis root cells. In red, localization of proteins at their steady state; in orange, trafficking proteins; red arrows, trafficking pathways from the SNX1 PVC/MVB endosome; green arrows, trafficking pathways toward the SNX1 PVC/MVB endosome. Note that PIN1 and part of PIN2 cycling are regulated by the GNoM protein during the GNoM-endosome-to-SNX1-endosome pathway, and by the VPS29 protein during the SNX1-endosome-to-PM pathway. GNL1, GNoM LIKE1; a1, VHAa1; GN, GNoM; Aleu, Aleurain.
The SNX1 MVB/PVC Endosome is Involved Both in the Endocytic and Secretory Pathways
The characterization of the Arabidopsis SORTING NEXIN1 (SNX1), a putative phosphatidyl inositol-3-phosphate binding protein, led us to the identification in Arabidopsis root cells of an endosomal compartment, designated SNX1 endosomes, which colocalizes with two homologues of the mammalian Rab5 protein, named RABF1 and RABF2b.7 Interestingly, another endosomal protein, GNOM,4,7 was not found to colocalize with SNX1, indicating that there are at least two distinct populations of endosomes in root cells, the SNX1 endosomes and the GNOM endosomes. Furthermore, we showed the SNX1 endosomes to be involved in the degradation of the auxin-efflux carrier PIN2 during the gravitropism response.7,23 Besides, BOR1, a PM boron transporter, also revealed to traffic through SNX1 endosomes on its way to the lytic vacuole, where it is degraded on high boron containing medium.6,11 Altogether, these data suggest that SNX1 endosomes are intracellular compartments involved in the degradation of PM proteins in the lytic vacuole following their internalization.
In BY-2 cells, the PVC/MVBs, which are labeled by the endocytic tracer FM4-64, are involved in the routing of vacuolar proteins to the vacuole.18 In Arabidopsis root tips, colocalization between SNX1 and PVC markers, as well as results of drug treatment experiments led us to conclude similarly that SNX1 endosomes are involved in the routing of vacuolar proteins.6 In tobacco protoplasts, the BP80 vacuolar sorting receptor (VSR) was shown to undergo a retrograde trafficking from the PVC back to the TGN.24 This finding was based on the effect of Wm, which inhibits the retrograde trafficking of VSRs leading to their depletion at the TGN and their routing to the vacuole.24 Interestingly, we found Wm to have basically the same effect in Arabidopsis root tips in inhibiting the retrograde trafficking of VSRs.6 Thus, our data support a role for the SNX1 PVC/MVB endosome in routing proteins coming from the endocytic or secretory pathway to the lytic vacuole or back to the TGN.6,7
The SNX1 PVC/MVB Endosome as a Recycling Compartment
We have recently found that the VACUOLAR PROTEIN SORTING29 (VPS29) is localized in SNX1 endosome/PVC/MVB in Arabidopsis roots.8 In the vps29 loss-of-function mutant, SNX1 PVC/MVB endosomes have an abnormal morphology, whereas other intracellular compartments, including the Golgi apparatus, TGN and GNOM endosomes have a wild-type shape phenotype.8 We found that in vps29, the continuous cycling of PIN1 was inhibited and that PIN1 then accumulated in the aberrant SNX1 PVC/MVB endosome. PIN1 is extremely stable at the PM and this stability is believed to depend on its continuous cycling.5 The observed inhibition of PIN1 recycling in vps29 led to PIN1 instability at the PM and its increased turnover.8 Therefore, the analysis of the vps29 mutant provides the first evidence for specific role of SNX1 PVC/MVB endosome in the endocytic recycling of a PM protein.
Altogether, our data indicate that in Arabidopsis roots, SNX1 PVC/MVB endosome can receive cargo proteins either from the endocytic or secretory pathway and then redirect them toward the vacuole, TGN or PM6–8 (Fig. 1B). We propose that this compartment is a major sorting platform in root cells to dynamically regulate the localization and quantity of membrane proteins and, thereby, is probably playing a key role in mediating cell polarity, cell signaling and cytokinesis.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5108
References
- 1.Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J, Gadella TW., Jr Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell. 2006;10:137–150. doi: 10.1016/j.devcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 5.Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 6.Jaillais Y, Fobis-Loisy I, Miege C, Gaude T. Evidence for a sorting endosome in Arabidopsis root cells. Plant J. 2008;53:237–247. doi: 10.1111/j.1365-313X.2007.03338.x. [DOI] [PubMed] [Google Scholar]
- 7.Jaillais Y, Fobis-Loisy I, Miege C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–109. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- 8.Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miege C, Gaude T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–1070. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 9.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 11.Takano J, Miwa K, Yuan L, von Wiren N, Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci U S A. 2005;102:12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter S, Geldner N, Schrader J, Wolters H, Stierhof YD, Rios G, Koncz C, Robinson DG, Jurgens G. Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature. 2007;448:488–492. doi: 10.1038/nature05967. [DOI] [PubMed] [Google Scholar]
- 13.Grebe M, Xu J, Mobius W, Ueda T, Nakano A, Geuze HJ, Rook MB, Scheres B. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr Biol. 2003;13:1378–1387. doi: 10.1016/s0960-9822(03)00538-4. [DOI] [PubMed] [Google Scholar]
- 14.Russinova E, Borst JW, Kwaaitaal M, Cano-Delgado A, Yin Y, Chory J, de Vries SC. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1) Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritzenthaler C, Nebenfuhr A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG. Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell. 2002;14:237–261. doi: 10.1105/tpc.010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teh OK, Moore I. An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature. 2007;448:493–496. doi: 10.1038/nature06023. [DOI] [PubMed] [Google Scholar]
- 17.Lam SK, Siu CL, Hillmer S, Jang S, An G, Robinson DG, Jiang L. Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell. 2007;19:296–319. doi: 10.1105/tpc.106.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004;16:672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, Friml J. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 20.Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc. 2004;214:159–173. doi: 10.1111/j.0022-2720.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- 21.Emans N, Zimmermann S, Fischer R. Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell. 2002;14:71–86. doi: 10.1105/tpc.010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G, Xue HW. Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response. Plant Cell. 2007;19:281–295. doi: 10.1105/tpc.106.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abas L, Benjamins R, Malenica N, Paciorek T, Wirniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8:249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 24.daSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J. Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell. 2005;17:132–148. doi: 10.1105/tpc.104.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von der Fecht-Bartenbach J, Bogner M, Krebs M, Stierhof YD, Schumacher K, Ludewig U. Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J. 2007;50:466–474. doi: 10.1111/j.1365-313X.2007.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fluckiger R, De Caroli M, Piro G, Dalessandro G, Neuhaus JM, Di Sansebastiano GP. Vacuolar system distribution in Arabidopsis tissues, visualized using GFP fusion proteins. J Exp Bot. 2003;54:1577–1584. doi: 10.1093/jxb/erg160. [DOI] [PubMed] [Google Scholar]