Abstract
Root-knot nematodes are plant parasitic worms that establish and maintain an intimate relationship with their host plants. RKN induce the redifferentiation of root cells into multinucleate and hypertrophied giant cells essential for nematode growth and reproduction. Major rearrangements of the cytoskeleton occur during giant cell formation. We characterized the first plant candidate genes implicated in giant cell actin and microtubule cytoskeleton reorganization. We showed previously that formins may regulate giant cell isotropic growth by controlling the assembly of actin cables. Recently we demonstrated that a Microtubule-Associated Protein, MAP65-3, is essential for giant cell development. In the absence of functional MAP65-3, giant cells started to develop but failed to fully differentiate and were eventually destroyed. In developing giant cells, MAP65-3 was associated with a novel kind of cell plate—the giant cell mini cell plate—that separates daughter nuclei. Despite karyokinesis occurs without cell division in giant cell, we demonstrated that cytokinesis is initiated and required for successful pathogen growth and development.
Key words: cytoskeleton, microfilament, formin, microtubule, microtubule-associated protein, giant cells, nematode
Root-knot nematodes (RKN) Meloidogyne spp. is one of the most damaging plant pathogen worldwide.1 Their potential host range encompasses more than 2,000 plant species. During a compatible interaction, these obligate biotrophic pathogen have evolved an ability to manipulate host functions to their own benefit.2–6 At the onset of parasitism, the infective second stage juveniles (J2) penetrate the root tip and migrate intercellularly to reach the root vascular cylinder. Each J2 then induces the redifferentiation of five to seven parenchymatic root cells into hypertrophied and multinucleate cells, named giant cells. Giant cells result from synchronous repeated karyokinesis without cell division.7 Despite lack of complete cytokinesis, we demonstrated that cytokinesis is initiated and essential for giant cell ontogenesis.8 Fully differentiated giant cells reach a final size about four hundred times that of root vascular cells and contain more than a hundred polyploid nuclei. Giant cell nuclei show an increase in DNA, possibly reflecting endoreduplication.9 These “feeding” giant cells constitute the exclusive source of nutrients for the nematode until reproduction. Giant cell development is accompanied by division and hypertrophy of surrounding cells, leading to a typical root gall formation, the primary visible symptom of infection.
Cytoskeleton Reorganization during Giant Cell Ontogenesis
The cytoskeleton plays a central role in cell differentiation, cell cycle and morphogenesis. Changes in cytoskeleton organization have been reported in various plant-microbe interactions.10–13 The distribution of microtubules (MT) and microfilaments in giant cells has recently attracted attention. Immunolocalization using actin and tubulin antibodies revealed that major rearrangements of the cytoskeleton occur during the formation of giant cells.14 Immunofluorescence microscopy of actin revealed thick actin cables going through the cytoplasm and cell cortex. In contrast, in the cytoplasm actin bundle segments are shorter, thinner and randomly oriented within an amorphous actin staining.
We characterized three FORMIN (FH) genes, AtFH1, AtFH6 and AtFH10, induced in the early stages of giant cell formation.15 Formins are actin-nucleating proteins that stimulate the de novo polymerization of actin filaments.16–19 We demonstrated that AtFH6 was anchored and uniformly distributed throughout the giant cell plasma membrane. We investigated the role of AtFH6 in cytoskeleton organization by determining that AtFH6 functionally suppressed the budding defect of a yeast formin mutant. Thus, these three formin genes may regulate giant cell isotropic growth by controlling the assembly of actin cables. During giant cell formation, these actin cables would guide the vesicle trafficking needed for extensive plasma membrane and cell wall biogenesis. Identification of formin interacting proteins in giant cells would allow the study of regulatory mechanisms and signaling molecules responsible for actin cytoskeleton reorganization.
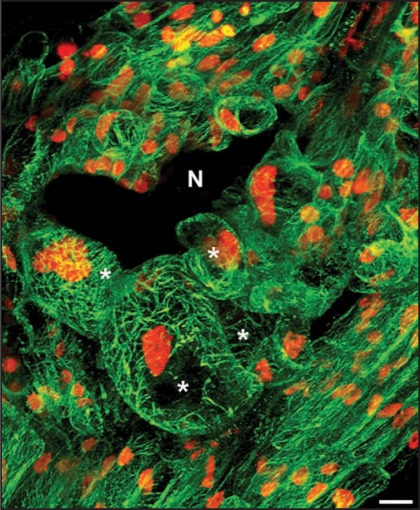
Recently, we characterized MT cytoskeleton organization in giant cell. The MAP4 MT-binding domain (MBD)-GFP reporter protein was used to visualize accurately dynamic changes in the organization of the MT cytoskeleton in living cells. Using in vivo confocal microscopy of gall section we revealed MT cortical arrays with bundling MTs in giant cells (Fig. 1). During cell cycle progression, microtubules reorganize into an anastral bipolar spindle, ensuring the accurate segregation of chromosomes during anaphase.20 Multiple spindles were observed in giant cells. Time-lapse in vivo revealed presence in mitotic giant cells of early synchronous phragmoplast arrays which do not further develop.8 In developing giant cells, phragmoplast restricted out-growth lead to the formation of a novel cell plate structure—the giant cell mini cell plate—that does not extend across adjacent faces of the cell. Optical and electron microscopy confirmed that giant cell mini cell plates, and subsequently mini cell walls, were frequently observed between two nuclei in giant cells. Detailed functional analyses of the Arabidopsis microtubule-associated protein MAP65-3 showed that this protein plays a critical role in giant cell development and is associated with the “mini cell plates” formed during cytokinesis initiation.8 We described an unparalleled defect in nematode feeding cell formation. In the absence of functional MAP65-3, giant cells started to develop but did not complete their differentiation and were eventually destroyed. These giant cell defects impaired the maturation of the infecting nematodes, which are dependent on the nutrients supplied by the giant cells. “Giant cell mini cell plates” were never observed in the absence of MAP65-3. Instead, aberrant cell wall stubs were observed in the first steps of T-DNA map65-3 mutant giant cell formation. Thus the “giant cell mini cell plate” may form a physical barrier separating the two daughter nuclei, required for the multiple rounds of mitosis that occur in developing giant cells, resulting in the formation of a functional feeding site. Defect in giant cell mini cell plate formationin the absence of MAP65-3 would lead to the accumulation of mitosis defects (cell wall stubs and connected nuclei) during repeated mitoses. These defects may prevent the development of functional feeding cells, resulting in the death of the nematode. Detailed functional analysis during plant development has highlighted the role of MAP65-3 in plant cell division. MAP65-3 plays a key role in MT arrays organization during both mitosis (spindle morphogenesis) and cytokinesis (phragmoplast expansion) in dividing plant cells. Thus, the nematode infection process provides a wonderful avenue to explore cell biological phenomena. Determining how a nematode modifies root cells to serve as feeding cells enhance our understanding of normal cell development.
Figure 1.
In vivo microtubule organization in developing giant cells induced by Meloidogyne incognita. Microtubule arrays in multinucleate giant cells and surrounding cells co-expressing microtubule-binding domain MBD-GFP and nuclear histone H2B:YFP proteins. N, nematode; Asterisks, giant cells. Bar = 10 µm.
Acknowledgements
This work was supported by INRA and GENOPLANTE contracts AF2001032 (“Identification of plant genes from the feeding sites induced by root-knot nematodes”) and ANR05GPLA020 “AFINDIS” (Arabidopsis Functions INvolved in DIsease Susceptibility). M.-C. C. was supported by a fellowship of the Ministère de l'Enseignement Supèrieur et de la Recherche.
Abbreviations
- FH
formin homology
- J2
infective second stage juveniles
- MAP
microtubule-associated protein
- MT
microtubules
- RKN
root-knot nematode
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5889
References
- 1.Trudgill DL, Blok VC. Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol. 2001;39:53–77. doi: 10.1146/annurev.phyto.39.1.53. [DOI] [PubMed] [Google Scholar]
- 2.Abad P, Favery B, Rosso MN, Castagnone Sereno P. Root-knot nematode parasitism and host response : molecular basis of a sophisticated interaction. Mol Plant Pathol. 2003;4:217–224. doi: 10.1046/j.1364-3703.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 3.Caillaud MC, Dubreuil G, Quentin M, Perfus Barbeoch L, Lecomte P, de Almeida Engler J, Abad P, Rosso MN, Favery B. Root-knot nematodes manipulate plant cell functions during a compatible interaction. J Plant Physiol. 2008;165:104–113. doi: 10.1016/j.jplph.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Gheysen G, Fenoll C. Gene expression in nematode feeding sites. Annu Rev Phytopathol. 2002;40:191–219. doi: 10.1146/annurev.phyto.40.121201.093719. [DOI] [PubMed] [Google Scholar]
- 5.de Almeida Engler J, Favery B, Engler G, Abad P. Loss of susceptibility as an alternative for nematode resistance. Current Opinion in Biotechnology. 2005;16:1–6. doi: 10.1016/j.copbio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Jammes F, Lecomte P, Almeida Engler J, Bitton F, Martin Magniette ML, Renou JP, Abad P, Favery B. Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. Plant J. 2005;44:447–458. doi: 10.1111/j.1365-313X.2005.02532.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones MGK, Payne HL. Early stages of nematode-induced giant cell formation in roots of Impatiens balsamina. J Nematol. 1978;10:70–84. [PMC free article] [PubMed] [Google Scholar]
- 8.Caillaud MC, Lecomte P, Jammes F, Quentin M, Pagnotta S, Andrio E, de Almeida Engler J, Marfaing N, Gounon P, Abad P, Favery B. MAP65-3 microtubule-associated protein is essential for nematode-induced giant cell ontogenesis in Arabidopsis. Plant Cell. 2008 doi: 10.1105/tpc.107.057422. www.plantcell.org/cgi/doi/10.1105/tpc.107.057422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiggers RJ, Starr JL, Price HJ. DNA content and variation in chromosome number in plant cells affected by Meloidogyne incognita and M. arenaria. Phytopathology. 1990;80:1391–1395. [Google Scholar]
- 10.Lipka V, Panstruga R. Dynamic cellular responses in plant-microbe interactions. Curr Opin Plant Biol. 2005;8:625–631. doi: 10.1016/j.pbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Hardham AR, Jones DA, Takemoto D. Cytoskeleton and cell wall function in penetration resistance. Curr Opin Plant Biol. 2007;10:342–348. doi: 10.1016/j.pbi.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Genre A, Chabaud M, Timmers T, Bonfante P, Barker DG. Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell. 2005;17:3489–3499. doi: 10.1105/tpc.105.035410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takemoto D, Hardham AR. The cytoskeleton as a regulator and target of biotic interactions in plants. Plant Physiol. 2004;136:3864–3876. doi: 10.1104/pp.104.052159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Almeida Engler J, Van Poucke K, Karimi M, De Groodt R, Gheysen G, Engler G. Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode-infected roots. Plant J. 2004;38:12–26. doi: 10.1111/j.1365-313X.2004.02019.x. [DOI] [PubMed] [Google Scholar]
- 15.Favery B, Chelysheva LA, Lebris M, Jammes F, Marmagne A, De Almeida-Engler J, Lecomte P, Vaury C, Arkowitz RA, Abad P. Arabidopsis formin AtFH6 is a plasma membrane-associated protein upregulated in giant cells induced by parasitic nematodes. Plant Cell. 2004;16:2529–2540. doi: 10.1105/tpc.104.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staiger CJ, Blanchoin L. Actin dynamics: old friends with new stories. Curr Opin Plant Biol. 2006;9:554–562. doi: 10.1016/j.pbi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Sagot I, Klee SK, Pellman D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat Cell Biol. 2002;4:42–50. doi: 10.1038/ncb719. [DOI] [PubMed] [Google Scholar]
- 18.Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Vavylonis D, Kovar DR, O'Shaughnessy B, Pollard TD. Model of formin-associated actin filament elongation. Mol Cell. 2006;21:455–466. doi: 10.1016/j.molcel.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasteneys G. Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci. 2002;115:1345–1354. doi: 10.1242/jcs.115.7.1345. [DOI] [PubMed] [Google Scholar]