Abstract
Plant growth and development are tightly regulated by both plant growth substances and environmental factors such as temperature. Taking into account the above, it was reasonable to point out that indole-3-acetic acid (IAA), the most abundant type of auxin in plants, could be involved in temperature- dependent growth of plant cells. We have recently shown that growth of maize coleoptile segments in the presence of auxin (IAA) and fusicoccin (FC) shows the maximum value in the range 30–35°C and 35–40°C, respectively. Furthermore, simultaneous measurements of growth and external medium pH indicated that FC at stressful temperatures was not only much more active in the stimulation of growth, but was also more effective in acidifying the external medium than IAA. The aim of this addendum is to determine interrelations between the action of IAA and FC (applied together with IAA) on growth and medium pH of maize coleoptile segments incubated at high temperature (40°C), which was optimal for FC but not for IAA.
Key words: auxin, fusicoccin, coleoptile segments, elongation growth, medium pH
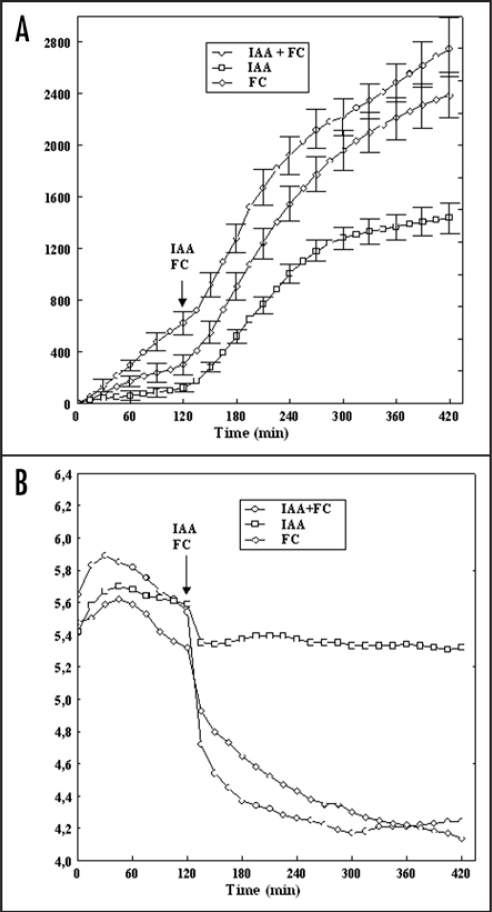
A well studied aspect of auxin action especially in maize coleoptile, is its effect on cell elongation, proton extrusion and membrane potential.1–7 It is now generally agreed that indole-3-acetic acid (IAA), as the principal regulator of plant elongation growth, causes (i) acceleration of elongation growth as compared to endogenous growth, (ii) enhancement of proton extrusion as compared to auxin—free medium, and (iii) transient depolarization followed by a slow hyperpolarization of membrane potential. According to the “acid growth theory” of elongation growth,8–11 auxin induced cell wall acidification provides favorable conditions for cell wall loosening, a requirement for cell elongation. At least in maize coleoptile segments, auxin induced cell wall acidification is mediated by increased activity and/or amount of the PM H+-ATPase.11,12 In the case of fusicoccin, which mimics the effect of auxin in many respects,13 it was shown that FC-binding site arises from interaction of the 14-3-3 protein dimmer with the C-terminal autoinhibitory domain of the H+-ATPase and that FC stabilizes this complex.14–18 It should be pointed out that in spite of abundant literature on the mechanism through which IAA or FC control growth of grass coleoptiles, little is know how these substances work at extreme temperatures. Over the past decade, the involvement of 14-3-3 proteins in plant stress responses has often been suggested.19 For example, work by Chelysheva et al.,20 and Babakov et al.,21 demonstrated that under low temperature and high osmolarity conditions, 14-3-3 proteins interact with the C-terminal autoinhibitory domain of the PM H+-ATPase activating the proton pump that play a key role in stress responses in higher plants. We have recently shown22 that FC at 40°C induced maximal growth whereas growth observed at the same temperature in the presence of IAA was reduced by 33% compared to the maximal value at 30°C. It was also found22 that at 40°C the kinetics of the pH change differed significantly for both growth substances; the segments treated with IAA at 40°C were virtually not able to acidify the external medium, whereas FC at this temperature caused practically maximal acidification. In this addendum we have shown that application of FC together with IAA conteracted the inhibitory effect of high temperature (40°C) on IAA-induced growth and proton extrusion in maize coleoptile segments (Fig. 1). For example, the total IAA-induced elongation growth of coleoptile segments at 40°C was 1438.1 ± 134.5 µm cm−1 (mean ± SE, n = 11) while elongation of 2747.4 ± 269.7 µm cm−1 (mean ± SE, n = 11) was observed in IAA applied together with FC (Fig. 1A). The data in Figure 1B indicate that coleoptile segments incubated at 40°C (over 2 h), without growth substances (control) characteristically changed the pH of the medium: usually within the first 30–45 min an increase of pH (by ca. 0.5 pH unit) was observed, followed by a slow decrease of pH. When IAA or FC was added (after 2 h of segment's incubation in control medium), an additional decrease of pH was observed. As can be seen in Figure 1B, FC added at 40°C was much more effective in acidification of the medium, as compared to IAA. For FC, 5h after its addition, the pH of the incubation medium dropped to pH 4.2, whereas for IAA the pH was only 5.4. However, addition of IAA together with FC at 40°C dropped medium pH approximately to the same value as was observed in the presence of FC only.
Figure 1.
Effect of high temperature (40°C) on growth (A) and medium pH (B) of maize coleoptile segments incubated in the presence of IAA (10 µM) and FC (1 µM). The growth of a stack of 21 segments, expressed as elongation (µm cm−1), was measured simultaneously with medium pH at 40°C. After preincubation (over 2 h) of the coleoptile segments in control medium, IAA and FC was added (arrow). Values are means of 11 independent experiments. Bars indicate ± SE. In the case of medium pH SE did not exceed 8%.
In conclusion, the results presented in this addendum provide further evidence that FC on the receptor level is much more effective than IAA.
Abbreviations
- IAA
indole-3-acetic acid
- FC
fusicoccin
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5896
References
- 1.Kutschera U, Schopfer P. Evidence against the acid-growth theory of auxin action. Planta. 1985;163:483–493. doi: 10.1007/BF00392705. [DOI] [PubMed] [Google Scholar]
- 2.Karcz W, Stolarek J, Pietruszka M, Malkowski E. The dose-response curves for IAA induced elongation growth and acidification of the incubation medium of Zea mays L. coleoptile segments. Physiol Plant. 1990;80:257–261. [Google Scholar]
- 3.Lüthen H, Bigdon M, Böttger M. Reexamination of the acid growth theory of auxin action. Plant Physiol. 1990;93:931–939. doi: 10.1104/pp.93.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felle H, Peters WS, Palme K. The electrical response of maize to auxins. Biochim Biophys Acta. 1991;1064:199–204. doi: 10.1016/0005-2736(91)90302-o. [DOI] [PubMed] [Google Scholar]
- 5.Karcz W, Stolarek J, Lekacz H, Kurtyka R, Burdach Z. Comparative investigation of auxin and fusicoccin-induced growth and H+-extrusion in coleoptile of Zea mays L. Acta Physiol Plant. 1995;17:3–8. [Google Scholar]
- 6.Claussen M, Lüthen H, Böttger M. Inside or outside? Localization of the receptor relevant to auxin-induced growth. Physiol Plant. 1996;98:861–867. [Google Scholar]
- 7.Karcz W, Burdach Z. A comparison of the effects of IAA and 4-Cl-IAA on growth, proton secretion and membrane potential in maize coleoptile segments. J Exp Bot. 2002;53:1089–1098. doi: 10.1093/jexbot/53.371.1089. [DOI] [PubMed] [Google Scholar]
- 8.Rayle DL, Cleland RE. Enhancement of wall loosening and elongation by acid solutions. Plant Physiol. 1970;46:250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayle DL, Cleland RE. The acid-growth theory of auxin induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hager A, Menzel H, Krauss A. Versuchte und Hypothese zur Primärwirkung des Auxins beim Streckungswachstum. Planta. 1971;100:1–15. doi: 10.1007/BF00386886. [DOI] [PubMed] [Google Scholar]
- 11.Hager A, Debus G, Edel HG, Stransky H, Serrano R. Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma membrane H+-ATPase. Planta. 1991;185:527–537. doi: 10.1007/BF00202963. [DOI] [PubMed] [Google Scholar]
- 12.Frias I, Caldeira MT, Pérez Castiñeira JR, Navarro Aviñó JP, Culiañez Maciá FA, Kuppinger O, Stransky H, Pagés M, Hager A, Serrano R. A major isoform of the maize plasma membrane H+-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell. 1996;8:1533–1544. doi: 10.1105/tpc.8.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrè E. Fusicoccin: a tool in plant physiology. Ann Rev Plant Physiol. 1979;30:273–288. [Google Scholar]
- 14.Jahn T, Fuglesang AT, Olsson A, Brüntrup IM, Collinge DB, Volkmann D, Sommarin M, Palmgren MG, Larsson C. The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H+-ATPase. Plant Cell. 1997;9:1805–1814. doi: 10.1105/tpc.9.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baunsgaard L, Fuglsang AT, Jahn T, Korthout HAAJ, De Boer AH, Palmgren MG. The 14-3-3 proteins associate with the plasma membrane H+-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant J. 1998;13:661–671. doi: 10.1046/j.1365-313x.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- 16.Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, Jensen ON, Aducci P, Palmgren MG. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr(946)-thr-Val and requires phosphorylation of Thr(947) J Biol Chem. 1999;274:36774–36780. doi: 10.1074/jbc.274.51.36774. [DOI] [PubMed] [Google Scholar]
- 17.Oecking C, Hagemann K. Association of 14-3-3 proteins with the C-terminal autoinhibitory domain of the plant plasma membrane H+-ATPase generates a fusicoccin-binding complex. Planta. 1999;207:480–482. [Google Scholar]
- 18.Würtele M, Jelich Ottmann Ch, Wittinghofer A, Oecking C. Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO J. 2003;22:987–994. doi: 10.1093/emboj/cdg104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts MR, Salinas J, Collinge DB. 14-3-3 proteins and the response to abiotic stress. Plant Mol Biol. 2002;1031:1031–1039. doi: 10.1023/a:1021261614491. [DOI] [PubMed] [Google Scholar]
- 20.Chelysheva VV, Smolenskaya IN, Trofimova MC, Babakov AV, Muromtsev GS. Role of the 14-3-3 proteins in the regulation of H+-ATPase activity in the plasma membrane of suspension-cultured sugar beet cells under cold stress. FEBS Lett. 1999;456:22–26. doi: 10.1016/s0014-5793(99)00923-0. [DOI] [PubMed] [Google Scholar]
- 21.Babakov AV, Chelysheva VV, Klychnikov OI, Zorinyanz SE, Trofimova MS, de Boer AH. Involvement of 14-3-3 proteins in the osmotic regulation of H+-ATPase in plant plasma membranes. Planta. 2000;211:446–448. doi: 10.1007/s004250000347. [DOI] [PubMed] [Google Scholar]
- 22.Karcz W, Burdach Z. Effect of temperature on growth, proton extrusion and membrane potential in maize (Zea mays L.) coleoptile segments. Plant Growth Regul. 2007;52:141–150. [Google Scholar]