Abstract
Reactive oxygen species (ROS) are commonly found in plants as natural by-products of the metabolism but their production is greatly enhanced under abiotic stresses. Particular metabolites and enzymes belonging to the ascorbate-glutathione cycle are able to scavenge these deleterious molecules and modulate the cellular redox-status. In the March issue of Journal of Plant Physiology, we have shown that drought stress induces a raise in glutathione reductase (GR) activity and gene expression that could be related to the intensity of the drought treatment and the drought susceptibility of the bean cultivar (cowpea and/or common bean). In the present addendum we show new data on GR specific activity during progressive drought stress and recovery of the drought-susceptible bean cultivar which can be related to the previously found dual-targeted GR gene expression. Furthermore, since in leguminous plants homoglutathione (hGSH) is generally the most abundant low molecular weight thiol form, we discuss on the occurrence of a (homo)glutathione reductase activity in beans.
Key words: common bean, cowpea, drought stress, (homo)glutathione, (homo)glutathione reductase, legumes, Phaseolus vulgaris, recovery, Vigna unguiculata
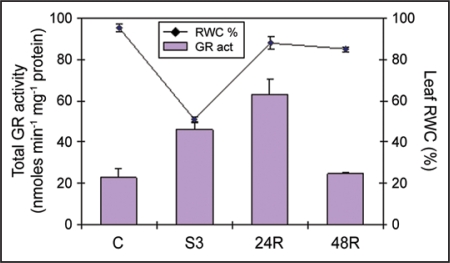
Drought stress is the most common form of abiotic stress and plants are likely to encounter periods of water shortage at least once in their lifecycle. One of the inevitable consequences of drought stress is enhanced reactive oxygen species (ROS) production which will imbalance the cellular redox-status. This shift in the steady-state cellular redox-status is currently believed to have an initial signaling effect, triggering adaptive/defense responses (reviewed in ref. 1). However, in order to avoid oxidative stress, enhanced ROS production must be kept under tight control by the cellular antioxidant machinery. Glutathione reductase (GR; EC 1.6.4.2) is a major cellular antioxidant enzyme. It belongs to the ascorbate-glutathione cycle and it is ubiquitously found in all cellular compartments.2 Using several bean plants (common bean, Phaseolus vulgaris and cowpea, Vigna unguiculata) as a model system to study drought responses and relate them to the degree of drought tolerance and/or susceptibility, we have shown that severe drought stress leads to an enhanced cellular GR activity related to the drought susceptibility of the cultivar.3,4 Similar results have also been found in a wheat system composed of drought-tolerant and susceptible cultivars.5 Regarding the more susceptible cultivar of our bean system (P.v. Carioca) and under severe drought stress (S3, Ψw = −2.0 MPa; RWC = 50.9%), total leaf GR activity was raised to approximately 200% when compared to control plants (C, Ψw = −0.5 MPa; RWC = 95.3%) (Fig. 1). This could translate a higher degree of oxidative stress due to enhanced ROS production in drought-susceptible cultivars than in drought-tolerant ones. In fact it has been shown that at the cellular level these drought-susceptible bean plants suffer a higher degree of membrane integrity loss when compared to the drought-tolerant.6–8 This can be related to enhanced ROS production since proteins and lipids of cellular membranes are main targets of ROS peroxidation.9
Figure 1.
GR-specific activity and relative water content (RWC%) in common bean (Phaseolus vulgaris) ‘Carioca’ leaves. GR-specific activity and RWC were measured in control, severely drought stressed and on rewatered plants. Values are means ± s.d. of three to five independent measurements. GR activity was assayed by following the oxidation of NADPH (decrease in absorbance at 340 nm) and expressed in nmoles min−1 mg−1 protein. RWC was measured according to Weatherley.24 Control plants (C), Ψw = −0.5 MPa; severely droughted plants (S3), Ψw = −2.0 MPa; 24 h rehydrated plant (24R), Ψw = −0.5 MPa; 48 h rehydrated plant (48R), Ψw = −0.5 MPa.
Considering the responses to drought at the whole-plant level, susceptible and tolerant beans also differ. In fact, drought-tolerant bean cultivars present a water-saving strategy by precocious control of stomatal opening which allows for photosynthetic activity to proceed at lower leaf Ψw.10–12 The maintenance of stomatal opening and photosynthetic activity during drought stress results in lower ROS production by photorespiration and/or the Mehler reaction as opposed to complete stomatal closure where inhibition of CO2 fixation occurs.1 Indeed, in the drought-tolerant cowpea cultivar, total GR activity was found constant throughout the progressive drought treatment.3
After 24 h rewatering (24R, Ψw = −0.5 MPa; RWC = 88.1%), from a moderate water stress: Ψw = −1.5 MPa; RWC = 69.2%, GR activity in the drought-susceptible bean cultivar was further raised by ∼270% as compared to control (Fig. 1). This enhanced GR activity can be directly related to the upregulation of the dual-targeted form of the bean GR gene (PvGRdt) (targeted to both chloroplasts and mitochondria) observed on rewatering of this drought-susceptible cultivar.4 In fact a significant upregulation of PvGRdt was detected as soon as 6 h after rewatering and the high expression levels were maintained up to 24 h after rewatering to then decrease at 48 h after rewatering.4 Hence in the drought-susceptible cultivar it seems that the dual-targeted form is more responsive to drought and rewatering than the cytosolic form. The same pattern was also seen on the less tolerant cowpea cultivar.3 Enhanced dual-targeted GR expression (and GR activity) under drought could be related to enhanced ROS production at those particular cellular compartments (mitochondria and chloroplasts). In fact, under a PEG-induced water deficit, drought-susceptible bean plants showed a higher number of disorganized chloroplasts when compared to the drought-tolerant,7 indicating that these organelles experienced oxidative stress during the treatment.
The GR enzyme is responsible for the reduction of glutathione disulfide (GSSG) to glutathione (GSH) using NADPH, and not only it keeps glutathione in the reduced state but it is also responsible for the maintenance of the cellular GSH:GSSG ratio.13,14 Interestingly, in leguminous plants such as the present bean plants, homoglutathione (hGSH) replaces completely or in part, glutathione (GSH). Homoglutathione has been shown to be the most abundant tripeptide in common bean and pea (Pisum sativum),15 in soybean (Glycine max),16 and in Lotus japonicus.17 The synthesis of hGSH proceeds through two ATP-dependent steps, the first step being common with GSH synthesis, the second step adding a β-Alanine instead of a Glycine to form the tripeptide. In the case of cowpea, which was up to now considered to be a non hGSH producing legume,15 we have recently detected the presence of a homoglutathione synthetase (hGSHS) mRNA and activity (MH Cruz de Carvalho, J Brunet, A Lameta, Y Zuily-Fodil and D Contour-Ansel, unpublished data).
Besides the chemical difference of the two thiol tripeptides, many of the roles ascribed to GSH are also performed by hGSH,18,19 particularly the control of the cellular redox status and ROS scavenging.20 However, the presence of hGSH questions on the occurrence of a homoglutathione reductase (hGR). A role for hGR as a detoxifying enzyme of the ascorbate-glutathione cycle has been suggested, using hGSSG (oxidized homoglutathione) instead of GSSG (oxidized glutathione), thus maintaining the cellular homoglutathione pool in the reduced state and acting as an antioxidative molecule in these plants.21,22 It can hence be suggested that in beans and other leguminous plants where both thiols co-exist (hGSH and GSH), the (h)GR enzyme will act as either a GR or a hGR in accordance to the thiol utilized in the ascorbate-glutathione cycle.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5918
References
- 1.Cruz de Carvalho MH. Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noctor G, Foyer C. Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 3.Contour Ansel D, Torres Franklin ML, Cruz de Carvalho MH, d'Arcy Lameta A, Zuily Fodil Y. Glutathione reductase in leaves of cowpea: cloning of two cDNAs, expression and enzymatic activity under progressive drought stress, desiccation and ABA treatment. Ann Bot. 2006;98:1279–1287. doi: 10.1093/aob/mcl217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres Franklin ML, Contour Ansel D, Zuily Fodil Y, Pham Thi AT. Molecular cloning of glutathione reductase cDNAs and analysis of GR gene expression in cowpea and common bean leaves during recovery from a moderate drought stress. J Plant Physiol. 2008;165:514–521. doi: 10.1016/j.jplph.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999;119:1091–1099. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasquez Tello A, Zuily Fodil Y, Pham Thi AT, Vieira da Silva J. Electrolyte and Pi leakages and soluble sugar content as physiological tests for screening resistance to water stress in Phaseolus and Vigna species. J Exp Bot. 1990;228:827–832. [Google Scholar]
- 7.Zuily Fodil Y, Vazquez Tello A, Vieira da Silva J. Effect of water deficit on cell permeability and on chloroplast integrity. Bulletin de la Société Botanique de France. 1990;137:115–123. [Google Scholar]
- 8.Roy Macauley H, Zuily Fodil Y, Kidric M, Pham Thi AT, Vieira da Silva J. Effect of drought stress on proteolytic activities in Phaseolus and Vigna leaves from sensitive and resistant plants. Physiol Plant. 1992;85:90–96. [Google Scholar]
- 9.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 10.Cruz de Carvalho MH, Laffray D, Louguet P. Comparison of the physiological responses of Phaseolus vulgaris and Vigna unguiculata cultivars when submitted to drought conditions. Environ Exp Bot. 1998;40:197–207. [Google Scholar]
- 11.Costa França MG, Thi ATP, Pimentel C, Rossiello ROP, Zuily Fodil Y, Laffray D. Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environ Exp Bot. 2000;43:227–237. doi: 10.1016/s0098-8472(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 12.Nunes C, Sousa Araujo S, Silva JM, Salema Fevereiro MP, Bernardes da Silva A. Physiological responses of the legume model Medicago truncatula cv. Jemalong to water deficit. Environ Exp Bot. 2008;63:289–296. [Google Scholar]
- 13.Pastori G, Foyer CH, Mullineaux P. Low temperature-induced changes in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves. J Exp Bot. 2000;51:107–113. [PubMed] [Google Scholar]
- 14.Noctor G, Gomez L, Vanacker H, Foyer CH. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot. 2002;53:1283–1304. doi: 10.1093/jexbot/53.372.1283. [DOI] [PubMed] [Google Scholar]
- 15.Matamoros MA, Moran JF, Iturbe-Ormaetxe I, Rubio MC, Becana M. Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiol. 1999;121:879–888. doi: 10.1104/pp.121.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klapheck S. Homoglutathione: isolation, quantification and occurrence in legumes. Physiol Plant. 1988;74:727–732. [Google Scholar]
- 17.Matamoros MA, Clemente MR, Sato S, Asamizu E, Tabata S, Ramos J, Moran JF, Stiller J, Gresshoff PM, Becana M. Molecular analysis of the pathway for the synthesis of thiol tripeptides in the model legume Lotus japonicus. Amer Phytopathol Soc. 2003;16:1039–1046. doi: 10.1094/MPMI.2003.16.11.1039. [DOI] [PubMed] [Google Scholar]
- 18.Zopes H, Klapheck S, Bergmann L. The function of homoglutathione and hydroxymethylglutathione for the scavenging of hydrogen peroxide. Plant Cell Physiol. 1993;34:515–521. [Google Scholar]
- 19.Frendo P, Jiménez MJH, Mathieu C, Duret L, Gallesi D, Van de Sype G, Hérouart D, Puppo A. A Medicago truncatula homoglutathione synthetase is derived from glutathione synthetase by gene duplication. Plant Physiol. 2001;126:1706–1715. doi: 10.1104/pp.126.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Nat Acad Sci USA. 1986;83:3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iturbe Ormaetxe I, Matamoros MA, Rubio MC, Dalton DA, Becana M. The antioxidants of legume nodule mitochondria. Amer Phytopath Soc. 2001;14:1189–1196. doi: 10.1094/MPMI.2001.14.10.1189. [DOI] [PubMed] [Google Scholar]
- 22.Naya L, Ladrera R, Ramos J, Gonzalez EM, Arrese Igor C, Minchin FR, Becana M. The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol. 2007;144:1104–1114. doi: 10.1104/pp.107.099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weatherley PE. Studies in the water relations of the cotton plant. I. The field measurement of water deficit in leaves. New Phytol. 1950;49:81–97. [Google Scholar]