Abstract
The signal transduction pathway of the plant stress and defense hormone, ethylene, has been extensively elucidated using the plant genetic model Arabidopsis over the last two decades. Among others, a MAPKKK CTR1 was identified as a negative regulator that has led to the speculation of MAPK involvement in ethylene signaling. However, it remained unclear how the MAPK modules acting downstream of the receptors to mediate ethylene signaling. We have recently presented new evidence that the MKK9-MPK3/6 modules identified by combined functional genomic and genetic screens mediate ethylene signaling, which is negatively regulated by the genetically identified CTR1-dependent cascades. Our genetic studies show consistently that the MKK9-MPK3/MPK6 modules act downstream of the ethylene receptors. Biochemical and transgenic analyses further demonstrated that the positive-acting and negative-acting MAPK activities are integrated and act simultaneously to control the key transcription factor EIN3 through dual phosphorylations to regulate the EIN3 protein stability and downstream transcription cascades. This study has revealed a novel molecular mechanism that defines the specificity of complex MAPK signaling. Comprehensive elucidation of MAPK cascades and the underlying molecular mechanisms would provide more precise explanations for how plant cells utilize MAPK cascades to control specific downstream outputs in response to distinct stimuli.
Key words: ethylene, EIN3, CTR1, MKK9, MPK3, MPK6
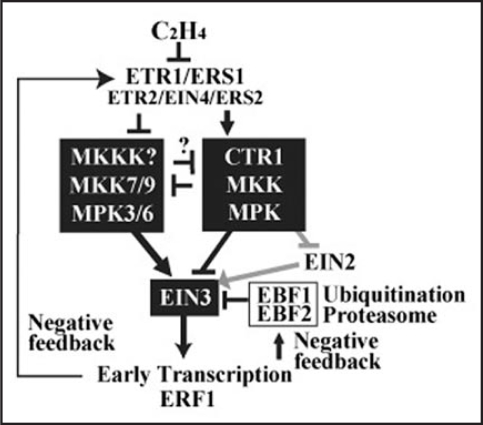
Ethylene (C2H4) regulates stress and defense responses and many key events of plant growth and development.1,2 In the plant model system Arabidopsis thaliana, several signaling components in the ethylene signal transduction pathway have been genetically identified from membrane receptors to nuclear activators as well as feedback regulators (Fig. 1).2,3 Multiple membrane proteins, ETR1, ETR2, ERS1, ERS2 and EIN4, play partially redundant roles as ethylene receptors.4–9 In the absence of ethylene, ETR1 and other receptors suppress hormone signaling by activating a negative regulator, CTR110,11 (a putative Raf-like MAPKKK), in ER complexes.12–15 Subsequently, a key transcription factor EIN3 is degraded by the 26S proteasome through the recognition by specific F-box proteins EBF1/2 in the E3 ligase complexes.16–20 Such action eventually blocks the downstream signal responses.
Figure 1.
Model of antagonistic MAPK cascades in ethylene signaling. A hypothetical MKKK is placed upstream of MKK7/9.
In contrast, upon binding of ethylene to the receptors, CTR1 is dissociated from receptor complexes and inactivated to initiate the downstream signaling.15 The immediately following principal nuclear event is to stabilize EIN3 proteins in the nucleus to activate the primary transcription.16–21 In ethylene signaling, a metal transporter-like membrane protein EIN2, that is genetically placed downstream of the receptors but upstream of EIN3, is to be important for the EIN3 accumulation.18,22 One of the primary EIN3 response genes with EIN3 binding sites in its promoter region, ERF1, is involved in the secondary transcription activation through its GCC element binding activity.23 There are several negative feedback mechanisms. For instance, ERS1 and ETR2 are transcriptionally elevated as a primary response to ethylene, and then the newly synthesized ethylene receptors attenuate the signaling effect of ethylene.6,8 EBF2 is also transcriptionally activated in early ethylene signaling and destabilizes EIN3 in the nucleus and diminish its accumulation.18
Despite the well-defined genetic pathway, cellular, molecular and biochemical connections among individual components remain to be elucidated in ethylene signaling. For example, an elevated level of MAPK-like activities have been reported in the loss-of-function ctr1 mutant, strongly implicating positively acting MAPKs are involved in ethylene signaling (Fig. 1).24,25 However, the MAPK cascade components have not been unequivocally identified.26
Here we have elucidated MAPK cascades in ethylene signaling using an integrative approach combining molecular, cellular, computational and genetic tools via exploring genomic information available in Arabidopsis.27,28 First, cell-based MAPK activity screen and ethylene-specific reporter assay facilitated by constitutively ethylene responsive ctr1 cells indicated the activity of antagonistic MAPK cascades in ethylene signaling: MKK9-MPK3/6 comprises positive-acting MAPK cascades, whereas CTR1 initiates negative-acting ones. Consistently, loss-of-function mkk9 shows a broad spectrum of ethylene insensitivity for the typical triple response, primary gene activation, ethylene-dependent growth inhibition and senescence promotion, as well as hypersensitivity to glucose and salt. The epistatic analysis using a transgenic approach indicates that MKK9 act downstream of the receptor complexes, but upstream or independent of EIN2. The MKK9 localization in the nucleus upon signaling as well as the MAPK cascade dependent EIN3 regulation in the nucleus have provided compelling evidence that two antagonistic MAPK cascades activities are integrated into regulating EIN3 through alternative phosphorylation, and modulating the protein stability and downstream transcription cascades. Significantly, this study establishes a new paradigm for linking complex MAPK cascades in controlling quantitative hormonal responses.
Since several hormone, stress and defense signals can activate MPK3 and MPK6 through upstream MAPK cascades in plants, it has long been questioned how converged MAPK signaling can secure their specificity. In this study, we have demonstrated that ethylene signaling specifically activates the MKK9-MPK3/6 modules that phosphorylate T174 of EIN3 and stabilize the EIN3 protein, but suppresses the CTR1-dependent cascades phosphorylating T592 of EIN3 that enhances the protein degradation. Only when both MAPK modules are regulated simultaneously, ethylene signaling can be appropriately executed in plants. This explains the broad but relatively weak ethylene insensitivity of mkk9 lacking only one part of two MAPK cascades involved in ethylene signaling. Likewise, mkk9 ctr1 double mutants displayed a partial but clear ethylene insensitivity in light-grown seedlings. Moreover, ctr1 displays a stronger constitutive ethylene signaling phenotype most likely due to the activation or derepression of the MKK9-MPK3/6 cascade in addition to the complete loss of CTR1-dependent MAPK cascade activity.
How CTR1 regulates the MKK9-MPK3/6 modules and which MKKs and MPKs are involved in the CTR1 modules remain to be determined. In our preliminary studies, there appears to be multiple MKKs sharing the activity of CTR1 in suppressing ethylene-specific reporter expression and promoting EIN3 degradation. Some loss- of-function mkk mutants exhibit ethylene hypersensitivity. The new studies have established essential cellular and genetics tools and assays as well as a novel conceptual foundation for more detailed molecular understanding of ethylene signaling. Future efforts will elucidate the complete and complex MAPK cascades in ethylene signaling as well as in other stress, defense and hormone signaling pathways.
Abbreviations
- MAPK
Mitogen activated protein kinase
- EIN3
ethylene insensitive3
- CTR1
constitutive triple response1
- MKK
MAPK kinase
- MKKK
MAPKK kinase
Footnotes
Previously published online as a Plant Signaling & behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5995
References
- 1.Abeles FB, Morgan PW, Saltveit JME. Ethylene in Plant Biology. 2nd edn. San Diego: Academic Press; 1992. [Google Scholar]
- 2.Schaller GE, Kieber JJ. Ethylene. In: Somerville C, Meyerowitz EM, editors. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2002. Vol. [DOI] [Google Scholar]
- 3.Alonso JM, Stepanova AN. The ethylene signalling pathway. Science. 2004;306:1513–1515. doi: 10.1126/science.1104812. [DOI] [PubMed] [Google Scholar]
- 4.Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitive to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- 5.Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene response gene ETR1: Similarity of product to two component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 6.Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene sensitivity conferred by Arabidopsis ERS gene. Science. 1995;269:1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- 7.Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:5812–5817. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- 10.Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Hall AE, O'Malley R, Bleecker AB. Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA. 2003;100:352–357. doi: 10.1073/pnas.0237085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 13.Clark KL, Larsen PB, Wang X, Chang C. Association of the Arabidopsis CTR1 raflike kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA. 1998;95:5401–5406. doi: 10.1073/pnas.95.9.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J. 2003;33:221–233. doi: 10.1046/j.1365-313x.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem. 2003;278:34725–34732. doi: 10.1074/jbc.M305548200. [DOI] [PubMed] [Google Scholar]
- 16.Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein Ethylene-Insensitive3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- 17.Yanagisawa S, Yoo SD, Sheen J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature. 2003;425:521–525. doi: 10.1038/nature01984. [DOI] [PubMed] [Google Scholar]
- 18.Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell. 2003;115:667–677. doi: 10.1016/s0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- 19.Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/s0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 20.Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra R. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA. 2004;101:6803–6808. doi: 10.1073/pnas.0401698101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD. The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell. 2007;19:509–523. doi: 10.1105/tpc.106.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–21452. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 23.Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITVE3 and ETHYLENE-ESPONSE-FACTOR1. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novikova GV, Moshkov IE, Smith AR, Hall MA. The effect of ethylene on MAP Kinase-like activity in Arabidopsis thaliana. FEBS. 2000;474:29–32. doi: 10.1016/s0014-5793(00)01565-9. [DOI] [PubMed] [Google Scholar]
- 25.Ouaked F, Rozhon W, Lecourieux D, Hirt H. A MAPK pathway mediates ethylene signaling in plants. EMBO J. 2003;22:1282–1288. doi: 10.1093/emboj/cdg131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ecker JR. Reentry of the ethylene MPK6 module. Plant Cell. 2004;16:3169–3173. [Google Scholar]
- 27.Yoo SD, Cho YH, Xiong Y, Tena G, Xiong Y, Sheen J. Dual control of nuclear EIN3 by bifurcated MAPK cascades in C2H4 signalling. Nature. 2008;451:789–795. doi: 10.1038/nature06543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nature Protocols. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]