Abstract
Oxidative stress, arising from an imbalance in the generation and removal of reactive oxygen species (ROS), is a challenge faced by all aerobic organisms. In plants, different pathways sense ROS from extracellular sources or organelles such as mitochondria, chloroplast or peroxisome. In our recent paper on Plant Molecular Biology1 we have studied the Arabidopsis thaliana early response to the generation of superoxide anion in chloroplasts during active photosynthesis. Transcript profile analysis revealed that the expression level of various genes encoding heat shock proteins (Hsps), increased after a short term of oxidative stress treatment. Furthermore, there was an induction of heat shock transcription factors HsfA2 and HsfA4A that were reported to be regulators of genes involved in stress response of Arabidopsis.1,2
In this addendum, we complement the expression analysis of two Hsp genes encoding Hsp70 and a 17.6 kDa class I small heat-shock protein (sHsp), and discuss their plausible role during oxidative stress, considering our data and other recently published papers.
Key words: heat shock factor, heat shock protein, chloroplast, oxidative stress, signalling
Abiotic stresses usually give rise to dysfunctional protein conformations. Molecular chaperones are stress proteins and many of them were originally identified as heat shock proteins (Hsps). According to current knowledge, Hsps facilitate protein refolding and stabilize polypeptides and membranes. Hsp70 has essential functions in preventing aggregation and assisting refolding of nonnative proteins under stress conditions.3 Small Hsps, however, are not able to refold nonnative proteins alone, but constitute complexes with unfolded proteins and other Hsps.3 Thus, the different classes of chaperones cooperate in cellular protection and play complementary and sometimes overlapping roles in the protection of proteins from stress.3
During photosynthesis, reactive oxygen species (ROS) generation occurs via electron transport reactions in the chloroplasts, such as the Mehler reaction, which generates superoxide anion that is converted to H2O2. ROS are also produced in the chloroplasts through the photoreduction of the herbicide methyl viologen (MV), which is a superoxide anion propagator. The relatively low reactivity of H2O2 suggests that it could diffuse from the chloroplast to initiate signaling events. Alternatively, the accumulation of H2O2 could be limited to chloroplasts initiating distinct signaling cascade.4 Environmental stresses like high light intensity, drought, extreme temperatures, heavy metals and UV radiations all enhance photosynthetic ROS generation. In the case of heat shock, there is emerging evidence that there is a cross-talk between heat and oxidative stress signaling. A burst of H2O2 was reported to occur after very short periods at high temperature, apparently as a result of NADPH oxidase activity.5 This burst has been correlated with the induction of heat responsive genes, a process assumed to be mediated through direct sensing of H2O2 by heat shock transcription factors (Hsfs).1,6
Analysis of our expression profile data showed that eighteen members of the Hsp family were highly induced, being nine of them small Hsps. Most of these genes were also responsive to high light treatment indicating that ROS originated in chloroplast could be the signaling molecules for Hsp expression.1 Recently published results show that 22 high light responsive genes, including Hsp, required photosynthetic electron transport for their expression.7
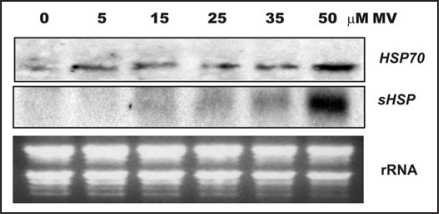
In this report we further analyzed the expression pattern of two MV highly responsive Hsp genes, Hsp70 (At3g12580) and Hsp17.6B-CI (At2g29500), under different stress situations to shed some light in the molecular mechanism connecting oxidative signals with cellular responses. These Hsp genes belong to two different gene families, Hsp70 is cytosolic and the subcellular location of Hsp17.6B-CI is unknown and both genes were induced by MV in a time-dependant manner.1 Added to this, Figure 1 reveals that the accumulation of these Hsp transcripts positively correlate with MV concentration. There is a basal level of Hsp70 transcripts, which rises when MV concentration is 50 µM whilst Hsp17.6B-CI expression occurs only under the most severe oxidative stress condition. These data suggest that Hsp genes from two distinct families respond differently to different levels of ROS.
Figure 1.
Northern blot analysis of the effect of MV concentration on the steady state level of transcripts encoding Hsp70 and sHSP. Two-week-old Arabidopsis plants were exposed to different MV concentrations for 2 h in the light. Twenty micrograms of total RNA from each sample were fractionated on formaldehyde-agarose gels, transferred to nylon membranes and hybridized with 32P-labelled Hsp70 or sHsp-specific DNA probes. Ethidium bromide stained rRNA was used as loading control.
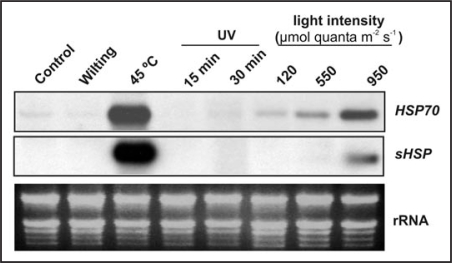
Hsp70 and Hsp17.6B-CI gene expression were followed under other abiotic stress sources such as wilting, heat shock, UV-light and different light intensities. There was a clear induction of Hsp70 and Hsp17.6B-CI in the case of high temperature or high light treatment, being the former three or four times higher than the latter. No change in transcript abundance was observed at short time in the case of wilting and UV treatments (Fig. 2).
Figure 2.
Effect of several stresses on transcript levels of Hsp70 and sHsp. Two-week-old Arabidopsis plants (control, 120 µmol quanta m−2 s−1, 16 h light period at 25°C) were exposed to wilting (2 h), heat shock (45°C, 2 h), UV-light (15 and 30 min) and different light intensities (120, 550 and 950 µmol quanta m−2 s−1 for 2 h). Transcript levels of Hsp70 and sHsp were detected using RNA gel-blot analysis. Each lane was loaded with 20 µg total RNA. Equal loading was verified by rRNA visualization after ethidium bromide staining.
Based on the present and previously reported data, it seems that a high degree of specialization exists in the response of specific Hsps to particular stress conditions, in a dose and time dependant manner.
The stress inducible expression of Hsps is regulated mainly at the transcriptional level by Hsfs that bind to conserved regulatory elements located in the promoters of Hsp genes, referred to as heat shock elements (HSEs).8 Two members of the Hsf family were highly induced by MV in our array: HsfA2 (At2g26150) and HsfA4A (At4g18880) while the expression pattern of other Hsfs was only slightly affected or remained essentially unchanged under this oxidative treatment.1 The eukaryotic HSEs with the palindromic consensus sequence nGAAn or nTTCn was highly represented in promoters of MV responsive Hsp genes such as Hsp70 (At3g12580), suggesting that this HSE could be functional in oxidative stress-specific transcription in Arabidopsis.1 Therefore, it seems likely that HsfA2 and HsfA4A proteins play an important role in the induction of defense system in response to oxidative stress. Moreover, HsfA4A is the principal candidate to function as a H2O2 sensor in Arabidopsis.6
The interaction between Hsp70 and Hsf has been suggested as a negative regulatory mechanism for Hsf-mediated transcriptional activation. Hsp70 may have a function in the attenuation of the transcriptional activity late in the heat shock response when excess Hsp70 is available and has the potential to bind to the activation domain of DNA bound HSF.9
Recent studies have revealed that several Hsps are redox regulated. The best studied is the prokaryotic Hsp33, which is induced by oxidative stress. Under reducing conditions Hsp33 is present in the cytosol as an inactive monomer. Oxidative stress will cause the intracellular redox environment to become more electropositive, favouring Hsp33 activation by dimerization through the formation of two intramolecular disulfide bonds.10 In plants, little is known about the redox regulation of Hsps. The deduced protein sequences of Hsps genes analyzed here are quite different in Cys contents. Thus, the cytosolic Hsp70 contains 7 Cys residues while they are absent in Hsp17.6B-CI, suggesting that only Hsp70 could suffer redox regulation. Further analysis of Hsp17.6B-CI subcellular location using TargetP prediction11 showed that it contains a putative chloroplastic transit peptide of 33 residues with a 0.479 score. Another sHsp, the tomato chloroplast HSP21, protects PSII from temperature-dependent oxidative stress.12 It is important to note that the expression of these two Hsp genes (At3g12580 and At2g29500) were not affected by apoplastic H2O2 or H2O2 produced by the plasma membrane,7 indicating that chloroplastic ROS contribute to the signaling cascade of these genes. The redox regulation of Hsps is matter of current research.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6021
References
- 1.Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol. 2008;66:361–378. doi: 10.1007/s11103-007-9274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schramm F, Ganguli A, Kiehlmann E, Englich G, Walch D, von Koskull-Doring P. The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol Biol. 2006;60:759–772. doi: 10.1007/s11103-005-5750-x. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Mullineaux PM, Karpinski S, Baker NR. Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol. 2006;141:346–350. doi: 10.1104/pp.106.078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desikan R, Hancock JT, Neill SJ. Oxidative stress signaling. In: Hirt H, Shinozaki K, editors. Plant responses to abiotic stress. Berlin Heidelberg: Springer-Verlag; 2004. pp. 73–93. [Google Scholar]
- 6.Miller G, Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann Bot. 2006;98:279–288. doi: 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechtold U, Richard O, Zamboni A, Gapper C, Geisler M, Pogson B, Karpinski S, Mullineaux PM. Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis. J Exp Bot. 2008;59:121–133. doi: 10.1093/jxb/erm289. [DOI] [PubMed] [Google Scholar]
- 8.Krishna P. Plant responses to heat stress. In: Hirt H, Shinozaki K, editors. Plant responses to abiotic stress. Berlin Heidelberg: Springer-Verlag; 2004. pp. 9–38. [Google Scholar]
- 9.Kim BH, Schöffl F. Interaction between Arabidopsis heat shock transcription factor 1 and 70 kDa heat shock proteins. J Exp Bot. 2002;53:371–375. doi: 10.1093/jexbot/53.367.371. [DOI] [PubMed] [Google Scholar]
- 10.Fedoroff N. Redox regulatory mechanisms in cellular stress responses. Ann Bot. 2006;98:289–300. doi: 10.1093/aob/mcl128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 12.Neta-Sharir I, Isaacson T, Lurie S, Weiss D. Dual role for tomato heat shock protein 21: protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. Plant Cell. 2005;17:1829–1838. doi: 10.1105/tpc.105.031914. [DOI] [PMC free article] [PubMed] [Google Scholar]