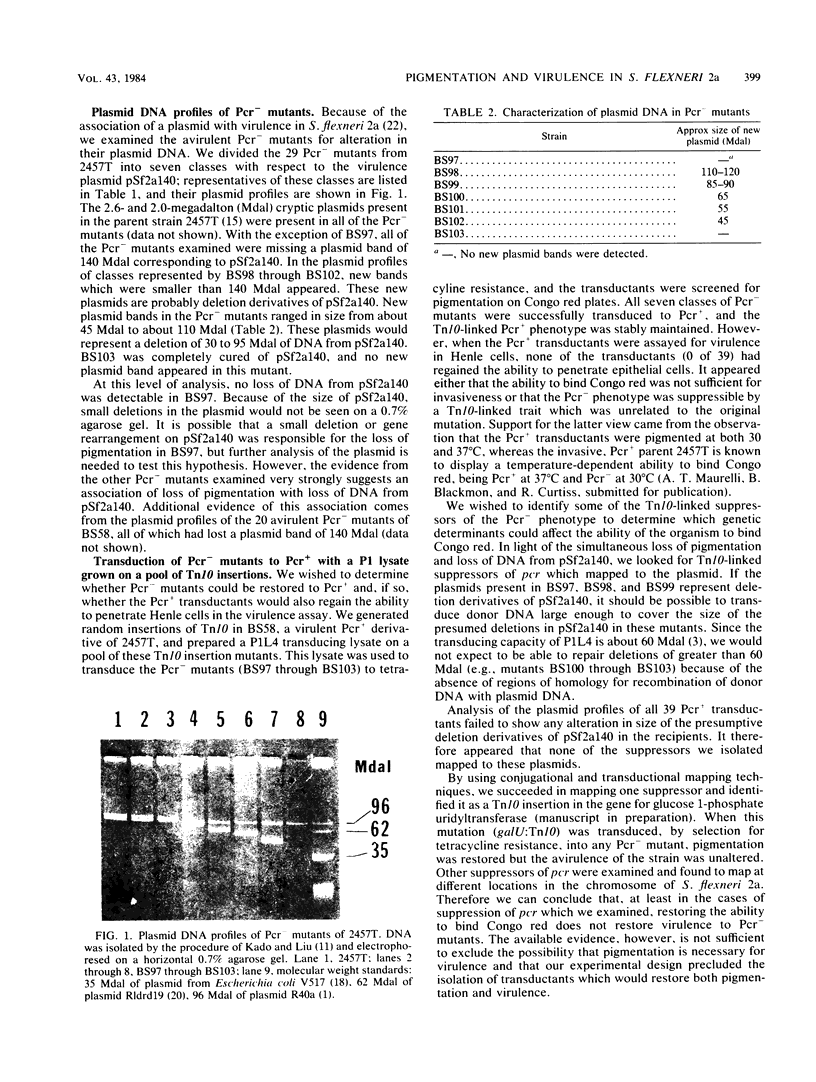
Abstract
In this study, we examined the relationship between the virulence of Shigella flexneri 2a and the ability of strains of S. flexneri 2a to absorb Congo red. Spontaneous nonpigmented (i.e., unable to bind Congo red [Pcr-]) derivatives of a virulent, pigmented (Pcr+) strain of S. flexneri 2a were isolated and assayed for virulence as determined by their ability to invade epithelial cells. All Pcr- mutants examined lost the ability to invade epithelial cells and were thus avirulent. Agarose gel electrophoresis of plasmid DNA from these avirulent, Pcr- mutants showed that the majority of these strains had lost a plasmid band corresponding to a virulence-associated plasmid, pSf2a140. In many of the mutants, concomitant loss of pigmentation, virulence, and pSf2a140 was accompanied by the appearance of a new plasmid, smaller than pSf2a140. We believe these new plasmids to be deletion derivatives of pSf2a140 and that loss of pigmentation and loss of virulence are associated with deletions in pSf2a140. We transduced Pcr- mutants to Pcr+ and isolated transductants which suppressed the Pcr- phenotype. None of the Pcr+ transductants regained the ability to invade epithelial cells. Several suppressors of the Pcr- phenotype were identified as mutations in cell wall biosynthesis. These results support our belief that although pigmentation is usually associated with virulence, genetic determinants unrelated to virulence can also affect the ability of the cell to bind Congo red. Therefore, the ability of S. flexneri 2a to bind Congo red does not necessarily imply the ability to invade epithelial cells. However, loss of ability to bind Congo red is accompanied by loss of virulence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOPER M. L., KELLER H. M., WALTERS E. W. Microscopic characteristics of colonies of Shigella flexneri 2a and 2b and their relation to antigenic composition, mouse virulence and immunogenicity. J Immunol. 1957 Mar;78(3):160–171. [PubMed] [Google Scholar]
- FORMAL S. B., DAMMIN G. J., LABREC E. H., SCHNEIDER H. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol. 1958 May;75(5):604–610. doi: 10.1128/jb.75.5.604-610.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Alexeichik J. A., Baron L. S. Behavior of coliphage lambda in Shigella flexneri 2a. J Virol. 1972 Oct;10(4):668–674. doi: 10.1128/jvi.10.4.668-674.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENLE G., DEINHARDT F. The establishment of strains of human cells in tissue culture. J Immunol. 1957 Jul;79(1):54–59. [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981 Apr;32(1):137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Holcombe J., Formal S. B. Molecular characterization of plasmids from virulent and spontaneously occurring avirulent colonial variants of Shigella flexneri. Infect Immun. 1979 May;24(2):580–582. doi: 10.1128/iai.24.2.580-582.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Mazigh D., Alonso J. M., Mollaret H. H. Simple method for demonstration of differential colony morphology of plasmid-associated virulent clones of Yersinia enterocolitica. J Clin Microbiol. 1983 Mar;17(3):555–557. doi: 10.1128/jcm.17.3.555-557.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A. Immunochemistry of Shigella flexneri O-antigens: a study of structural and genetic aspects of the biosynthesis of cell-surface antigens. Bacteriol Rev. 1971 Jun;35(2):117–148. doi: 10.1128/br.35.2.117-148.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]