Abstract
Many species of rhizobial bacteria can invade their plant hosts and induce development of symbiotic nitrogen-fixing nodules only if they are able to produce an acidic exopolysaccharide (EPS) with certain structural and molecular weight characteristics.1–3 Sinorhizobium meliloti that produces the functional form of the exopolysaccharide succinoglycan induces formation of invasion structures called infection threads in the root hair cells of its plant hosts alfalfa and Medicago truncatula. However, S. meliloti mutants that cannot produce succinoglycan are not able to induce infection thread formation, resulting in an early arrest of nodule development and in nitrogen starvation of the plant. Mounting evidence has suggested that succinoglycan acts as a signal to these host plants to permit the entry of S. meliloti. Now, our microarray screen and functional category analysis of differentially-expressed genes show that M. truncatula plants inoculated with wild type S. meliloti receive a signal to increase their translation capacity, alter their metabolic activity and prepare for invasion, while those inoculated with a succinoglycan-deficient mutant do not receive this signal, and also more strongly express plant defense genes.
Key words: nitrogen fixation, nodule, succinoglycan, microarray, legume, rhizobial bacteria, Sinorhizobium meliloti, Medicago truncatula, infection thread, root hair
Introduction
An infection thread is a progressive ingrowth of the host plant root hair cell membrane that is induced by a compatible strain of rhizobial bacteria.4 It is through these structures that the bacteria reach the interior of the root where they are endocytosed by host cells and differentiate into the nitrogen-fixing bacteroid form, establishing a nitrogen-fixing symbiosis.4 Infection threads are formed after a microcolony of bacteria has been trapped by the tight curling of the root hair in response to Nod factor (NF) (Fig. 1), the first rhizobially-produced signal known to interact with the plant host during symbiotic development.4 Infection thread formation is hypothesized to be an inversion of the outward-directed polar tip growth normally exhibited by root hairs, and to require similar cytoskeletal rearrangements, new cell membrane synthesis, and deposition of cell wall material.5 M. truncatula root hairs inoculated with a succinoglycan-deficient exo Y mutant of S. meliloti fail to form infection threads although they respond to the normal NF produced by this mutant strain, forming a colonized curled root hair.6
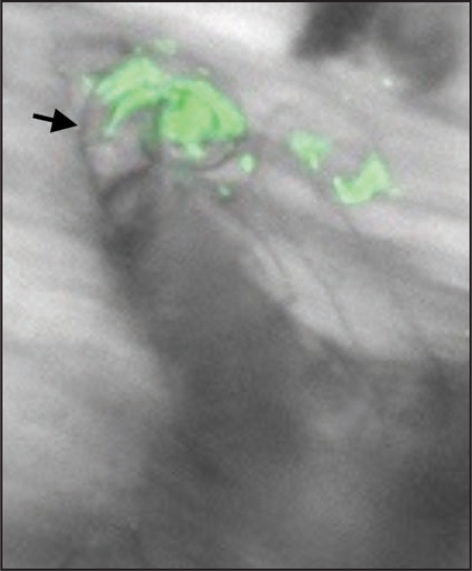
Figure 1.
An infection thread (arrow) elongating in an M. truncatula root hair, and populated with GFP-expressing S. meliloti.
To begin to dissect the early reactions of M. truncatula to the succinoglycan signal, we examined the differential gene expression responses of this plant host to succinoglycan-producing vs. succinoglycan-deficient S. meliloti at three days post-inoculation (dpi). At this early time point colonized curled root hairs have formed, but infection threads have not yet initiated. Therefore, the observed differential gene expression responses are likely due to the plant's reaction to succinoglycan itself, rather than to later events such as infection thread abortion or nitrogen starvation. Our data, obtained using microarrays representing 16,000 M. truncatula genes, show that plants inoculated with wild type, succinoglycan-producing S. meliloti more strongly express genes encoding translation components, protein degradation machinery, some nodulins, and components of plastids and the photosynthetic apparatus than plants inoculated with the succinoglycan-deficient mutant. In contrast, M. truncatula inoculated with the succinoglycan-deficient mutant more strongly express an unexpectedly large number of plant defense genes and genes of unknown function. Lohar et al., (2006) observed that between 1 and 3 dpi, genes encoding translation apparatus components are induced in roots inoculated with wild type S. meliloti relative to uninoculated roots.7 Our results show a similar difference in the expression of translation-related genes between plants inoculated with the wild type vs. the succinoglycan-deficient mutant, suggesting that the succinoglycan signal is responsible for this increase in translation capacity.
Possible Mechanisms of Succinoglycan Function
How might the M. truncatula roots perceive succinoglycan? One possibility is that succinoglycan is recognized by a plant receptor. Recognition of structural elements of symbiotic exopolysaccharides by Medicago species is implied by the ability of a particular exopolysaccharide to permit invasion by S. meliloti on some of its plant hosts but not on other hosts. Succinoglycan is the only S. meliloti exopolysaccharide that can induce infection thread formation on the host M. truncatula, however either succinoglycan or a second S. meliloti exopolysaccharide, galactoglucan, can induce infection thread formation on the host Medicago sativa (alfalfa).3 Also, transfer of the genes required for succinoglycan production from S. meliloti 1021 to S. meliloti Rm41 confers on this strain the ability to form functional nodules on M. truncatula ecotype A17.8 Possible candidates for a succinoglycan receptor are two genes shown in our study to be expressed more strongly in M. truncatula roots inoculated with wild type S. meliloti than in those inoculated with the succinoglycan-deficient mutant: a putative β-glucan receptor (M. truncatula consensus EST TC104170) and a leucine-rich-repeat receptor (TC103114). Such upregulation of the expression of a gene encoding a succinoglycan receptor would increase the supply of receptor available for insertion in the new membrane material at the growing tip of the infection thread. A β-glucan receptor with a similar function is found in soybean where it recognizes a hepta-β-glucoside from the fungal pathogen Phytophthora sojae, which elicits defense responses in this plant.9,10 Cyclic 1,3-1,6-β-glucans from the soybean symbiont Bradyrhizobium japonicum have been implicated in suppressing these defense responses.9
Succinoglycan also appears to have an effect on M. truncatula defense responses. A significant number of plant defense genes are among those expressed more strongly in plants inoculated with succinoglycan-deficient S. meliloti suggesting that either succinoglycan suppresses basal expression levels of these genes in M. truncatula or that plant contact with S. meliloti in the absence of succinoglycan induces their expression. Previous studies had found morphological markers of plant defense such as auto-fluorescent phenolic compounds and the defensive cell wall material callose associated with failed infection threads induced by succinoglycan-deficient S. meliloti.11 However, our gene expression results show evidence of a plant defense response to succinoglycan-deficient S. meliloti at 3 dpi, which predates infection thread failure. Lohar et al., found evidence for suppression of a plant defense response during invasion by wild type S. meliloti.7 That study showed that within 1 hr of inoculation with wild type S. meliloti, a significant number of M. truncatula plant-defense genes are upregulated, however, by 2–3 dpi this induction has disappeared.7 Taken together, these results suggest that roots inoculated with the succinoglycan-deficient mutant are arrested at this early, defensive stage. Succinoglycan might play a role in suppressing this defense response.
Several lines of evidence suggest that it is the low-molecular-weight (LMW) forms of symbiotic exopolysaccharides that function in symbiosis.1,6,12 One possible reason for the requirement for LMW exopolysaccharide might be that only LMW forms can reach the root-hair cell membrane to deliver a signal whereas the plant cell wall prevents the access of HMW forms to the cell membrane. Another possibility is that LMW succinoglycan has a greater capacity than the HMW form to modulate the chemical properties of the interior of the infection thread. Root hair tip growth is profoundly affected by factors such as pH, Ca2+ ion concentration, and reactive oxygen species (ROS)13—some of the same factors that may be affected by acidic exopolysaccharides.
For example, Qi et al., (2005) found that LMW fractions of the algal polysaccharide ulvan had greater reducing power and a greater ability to chelate metals such as Fe2+, which might be due to the greater concentration of reducing and non-reducing saccharide ends in the LMW fractions.14 These authors speculate that the reducing power and metal chelation capacity of LMW ulvan might prevent the production of some ROS species.14 An appropriate level of ROS in the infection thread may be necessary for cross-linking of the plant cell wall matrix material and contributing to the progressive ingrowth of the thread,15,16 but an excess of ROS could be detrimental.
One theory is that bacterial exopolysaccharides from plant pathogenic bacteria modulate host calcium signaling.17 These bacterial EPSs might exert this effect by serving as an extracellular Ca2+ sink and reducing the Ca2+ that is available for influx into plant cells.17 Since Ca2+ flux is central to the initial signaling pathway of NF in M. truncatula roots,18 it is also possible that rhizobial exopolysaccharides reduce the free Ca2+ levels in the interior of the infection thread, modulating the effects of the NF signal during the bacterial invasion process.
An important implication of our work is that switching the host range of rhizobial species may require the transfer not only of the genes required for NF biosynthesis between rhizobial species, but also those required for exopolysaccharide synthesis. It has already been shown that transfering the genes encoding NF receptors between plant species can be insufficient to switch symbiont range. Transformed M. truncatula roots expressing the Lotus japonicus NF receptor genes LjNfr1 and LjNfr5 permitted infection thread initiation by the L. japonicus symbionts Mesorhizobium loti or Rhizobium leguminosarum bv. viciae strain DZL, but these infection threads aborted in, respectively, the root epidermis or the nodule primordium.19
Further dissection of legume plant responses to rhizobial exopolysaccharides using genomic, genetic and cytological techniques should ultimately reveal the mechanisms by which these compounds induce infection thread formation and facilitate symbiotic development.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6512
References
- 1.Staehelin C, Forsberg LS, D'Haeze W, Gao MY, Carlson RW, Xie ZP, Pellock BJ, Jones KM, Walker GC, Streit WR, Broughton WJ. Exo-oligosaccharides of Rhizobium sp. strain NGR234 are required for symbiosis with various legumes. J Bacteriol. 2006;188:6168–6178. doi: 10.1128/JB.00365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leigh JA, Reed JW, Hanks JF, Hirsch AM, Walker GC. Rhizobium meliloti mutants that fail to succinylate their Calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell. 1987;51:579–587. doi: 10.1016/0092-8674(87)90127-9. [DOI] [PubMed] [Google Scholar]
- 3.Glazebrook J, Walker GC. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989;56:661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- 4.Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat Rev Microbiol. 2007;5:619–633. doi: 10.1038/nrmicro1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gage DJ. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev. 2004;68:280–300. doi: 10.1128/MMBR.68.2.280-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng HP, Walker GC. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J Bacteriol. 1998;180:5183–5191. doi: 10.1128/jb.180.19.5183-5191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohar DP, Sharopova N, Endre G, Penuela S, Samac D, Town C, Silverstein KA, VandenBosch KA. Transcript analysis of early nodulation events in Medicago truncatula. Plant Physiol. 2006;140:221–234. doi: 10.1104/pp.105.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simsek S, Ojanen-Reuhs T, Stephens SB, Reuhs BL. Strain-ecotype specificity in Sinorhizobium meliloti-Medicago truncatula symbiosis is correlated to succinoglycan oligosaccharide structure. J Bacteriol. 2007;189:7733–7740. doi: 10.1128/JB.00739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mithöfer A, Bhagwat AA, Feger M, Ebel J. Suppression of fungal β-glucan-induced plant defence in soybean (Glycine max L.) by cyclic 1,3-1,6-β-glucans from the symbiont Bradyrhizobium japonicum. Planta. 1996;199:270–275. [Google Scholar]
- 10.Mithöfer A, Fliegmann J, Neuhaus-Url G, Schwarz H, Ebel J. The hepta-beta-glucoside elicitor-binding proteins from legumes represent a putative receptor family. Biol Chem. 2000;381:705–713. doi: 10.1515/BC.2000.091. [DOI] [PubMed] [Google Scholar]
- 11.Niehaus K, Kapp D, Puhler A. Plant defence and delayed infection of alfalfa pseudonodules induced by an exopolysaccharide (EPS I)-deficient Rhizobium meliloti mutant. Planta. 1993;190:415–425. [Google Scholar]
- 12.Jones KM, Walker GC. >Critical differences in nodule invasion phenotypes of S. meliloti mutants in succinoglycan production between the plant hosts M. truncatula and alfalfa. manuscript in preparation. [Google Scholar]
- 13.Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi H, Zhao T, Zhang Q, Li Z, Zhao Z, Xing R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta) Journal of Applied Phycology. 2005;17:527–534. [Google Scholar]
- 15.Jamet A, Mandon K, Puppo A, Herouart D. H2O2 is required for optimal establishment of the Medicago sativa/Sinorhizobium meliloti symbiosis. J Bacteriol. 2007;189:8741–8745. doi: 10.1128/JB.01130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewin NJ. Plant cell wall remodelling in the Rhizobium-Legume symbiosis. Critical Reviews in Plant Sciences. 2004;23:293–316. [Google Scholar]
- 17.Aslam SN, Jackson RW, Morrissey K, Knight MR, Chinchilla D, Boller T, Erbs G, Tandrup Jensen T, Newman MA, Cooper RM. Bacterial extracellular polysaccharides promote pathogenicity by suppressing plant basal defences. In: Lorito M, Scala F, editors. The XIII International Congress on Molecular Plant-Microbe Interactions Sorrento. Italy: The American Phytopathological Society; 2007. in press. [Google Scholar]
- 18.Oldroyd GE, Downie JA. Nuclear calcium changes at the core of symbiosis signalling. Curr Opin Plant Biol. 2006;9:351–357. doi: 10.1016/j.pbi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Radutoiu S, Madsen LH, Madsen EB, Jurkiewicz A, Fukai E, Quistgaard EM, Albrektsen AS, James EK, Thirup S, Stougaard J. LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. Embo J. 2007;26:3923–3935. doi: 10.1038/sj.emboj.7601826. [DOI] [PMC free article] [PubMed] [Google Scholar]