Abstract
Plants possess a unique metabolic diversity commonly designated as secondary metabolism, of which the anticancer alkaloids from Catharanthus roseus are among the most studied. Recently, in a classical function-to-protein-to-gene approach, we have characterized the main class III peroxidase (Prx) expressed in C. roseus leaves, CrPrx1, implicated in a key biosynthetic step of the anticancer alkaloids. We have shown the vacuolar sorting determination of CrPrx1 using GFP fusions and we have obtained further evidence supporting the role of this enzyme in alkaloid biosynthesis, indicating the potential of CrPrx1 as a molecular tool for the manipulation of alkaloid metabolism. Here, we discuss how plant cells may regulate Prx reactions. In fact, Prxs form a large multigenic family whose members accept a broad range of substrates and, in their two subcellular localizations, the cell wall and the vacuole, Prxs co-locate with a large variety of secondary metabolites which can be accepted as substrates. How then, are Prx reactions regulated? Localization data obtained in our lab suggest that arabinogalactan proteins (AGPs) and Prxs may be associated in membrane microdomains, evocative of lipid rafts. Whether plasma membrane and/or tonoplast microcompartmentation involve AGPs and Prxs and whether this enables metabolic channeling determining Prx substrate selection are challenging questions ahead.
Key words: class III peroxidases, CrPrx1, indole alkaloids, vacuole, secondary metabolites, arabinogalactan proteins, lipid rafts
A Class III Peroxidase and the Anticancer Alkaloids from Catharanthus roseus
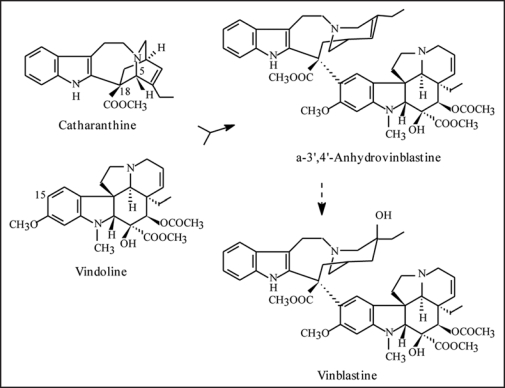
Catharanthus roseus (L.) G. Don is the source of the first natural drugs used in cancer therapy, the dimeric terpenoid indole alkaloids vinblastine and vincristine, produced in very low levels in the leaves of the plant. Since early studies of the biosynthesis of the anticancer alkaloids, the dimerization reaction leading to α-3∼,4∼-anhydrovinblastine (AVLB, Fig. 1) has attracted much attention due to its possible regulatory importance and potential application, either for increasing the anticancer alkaloid levels in planta, or for the semi synthetic production of the natural anticancer alkaloids and their derivatives. Initial work with cell suspension cultures suggested the involvement of peroxidase-like enzymes in AVLB biosynthesis, but remained largely inconclusive since the dimeric alkaloids do not accumulate in cultured cells.1–3 In our labs, we have conducted a systematic search for AVLB synthase activity in the leaves of the plant, where both the monomeric precursors and the dimeric products accumulate. After partial purification, a peroxidase-like dimerization activity was unmasked and ascribed to the single class III peroxidase (Prx) isoenzyme activity detected in C. roseus leaves.4 This activity was purified to homogeneity revealing a protein with all the characteristics of Prxs, CrPrx1, and subcellular localization studies pointed to its localization in the vacuole, where the monomeric alkaloid precursors and dimeric products are also located.5,6
Figure 1.
Biosynthesis of vinblastine from the monomeric precursors catharanthine and vindoline. Anhydrovinblastine is the product of the dimerization reaction and the precursor of the anticancer drugs vinblastine and vincristine.
In a classical function-to-protein-to-gene approach published recently in Plant Physiology (February 2008; vol 146:403–17),7 we have purified CrPrx1, the main Prx and single AVLB synthase activity found in C. roseus leaves, we have obtained a partial aminoacid sequence which was used to isolate the CrPrx1 gene by a RT-PCR based strategy, and we have obtained further evidence confirming the vacuolar localization of Crprx1 and supporting its role in AVLB biosynthesis.
CrPrx1 is encoded by a single copy gene with two introns and it contains all conserved and highly conserved residues typical of Prxs, including the two His residues interacting with the heme and the eight Cys residues forming four disulfide bridges. N-terminal aminoacid sequencing of the purified CrPrx1 enabled the identification of an N-terminal propeptide of 34 amino acids, which is predicted to be a signal peptide to the secretory system and was indeed able to direct a GFP fusion to the ER. Moreover, alignment of the full deduced sequence of CrPrx1 with previously characterized Prxs indicated the presence of a C-terminal extension with 23 to 25 amino acids, which was shown to be essential for vacuolar sorting of a GFP-Crprx1 fusion, and also confirmed the vacuolar localization of Crprx1. Likewise, phylogenetic analysis indicated that CrPrx1 belongs to an evolutionary branch of vacuolar Prxs that likely preceded the divergence between monocots and dicots, and whose members appear to have been recruited for different functions during evolution. On the other hand, an Arabidopsis vacuolar Prx from a different phylogenetic branch seems to have the same expression profile as CrPrx1, indicating that Prxs may be replaceable during evolution, in line with the largely overlapping reactivity properties they show.
Expression and activity studies further supported a role for CrPrx1 in indole alkaloid biosynthesis, although a correlation between CrPrx1 and alkaloid levels was not always found. This may be a consequence of the indole alkaloid pathway being dependent on a complex network of factors and regulation programs, but also a consequence of the regulation of CrPrx1 activity by the availability of H2O2. In fact, the source of H2O2 still remains one of the main mysteries concerning the oxidative activity of Prxs. As a whole, this work is one of the few thorough characterizations of a vacuolar class III peroxidase and its possible function, and has produced a molecular tool which may be of use to increase the anticancer alkaloid levels in the plant, or to produce a biotransformation system suitable for the large scale production of the dimeric anticancer alkaloids and their derivatives from the in planta abundant precursors vindoline and catharanthine (Fig. 1).
Class III Peroxidases and Secondary Metabolites—Who Meets Who?
This crossroads between a Prx and the biosynthesis of a class of secondary metabolites is interesting because both Prxs and secondary metabolism are paradigms of the phenotypic plasticity of plants and are, in many ways, two sides of the same coin. Prxs form large multigenic families in plant species, and they accept a broad range of substrates, showing largely overlapping reactivities. Thus, Prxs represent a case of high functional redundancy, which makes the characterization of their functions a daunting and frequently intangible task. Likewise, secondary metabolism is extremely prodigal in the plant kingdom and their products seem to have redundant roles, since several compounds with similar effects may be produced by the same plant tissue, and they have low specificity, since the same compound seems to be active in a number of different situations. Again, as for Prxs, this makes it very difficult to draw conclusions about the roles of secondary metabolites, which remain largely uncertain. Thus, in plants, evolution seems to have often selected broad range survival strategies with low specificity, of which secondary metabolites and class III peroxidases are prime examples.
But the crossroads between Prxs and the biosynthesis of secondary metabolites raises also several recurring questions. In their two subcellular localizations, the cell wall and the vacuole, Prxs co-locate with a large variety of secondary metabolites which also accumulate preferentially in these two compartments, and which can be accepted as Prx substrates, including aromatic phenols, amines, indoles, alkaloids, sulfonates, etc.8 How then, are Prx reactions regulated? Are they just chance metabolic plasticity? Is spatial and temporal co-localization with the most concentrated/higher affinity substrate determining who wins the race? Or could some sort of metabolic channeling play a decisive role?
Class III Peroxidases and Membrane Microdomains Containing Arabinogalactan Proteins—Unlikely Partners Determining Peroxidase-Mediated Secondary Metabolism Reactions?
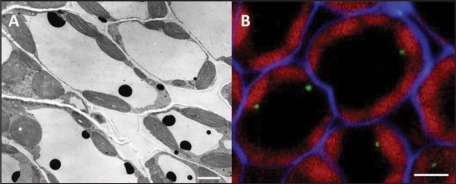
In C. roseus, CrPrx1 localizes at the inner face of the tonoplast with a punctuated distribution suggesting a meaningful spatial organization (Fig. 2A). This has led us to propose a metabolic channeling model for the biosynthesis of AVLB in which CrPrx1 may be localized in specific microdomains of the tonoplast near the putative vindoline and catharanthine transporter,9 in such a way that alkaloid transport through the tonoplast would be coupled to its immediate oxidation by CrPrx1.6 We further proposed that the generation of H2O2 could be mediated by a tonoplast NADPH oxidase co-localized with CrPrx1 and the alkaloid transporter.6 The proposed model, in which several proteins cooperate in specific membrane localizations, correlates interestingly with the recent set of data indicating the existence in plant plasma membranes of sterol-rich, detergent-resistant microdomains called lipid rafts. These microdomains are proposed to host glycosyl-phosphatidylinositol(GPI)-anchored proteins and a subset of integral and peripheral cell surface proteins which interact to perform specific functions. Among GPI-anchored proteins, plants possess a particularly prominent and plant specific group—arabinoglactan proteins (AGPs). AGPs are complex proteoglycans found at the surface of all plant cells and they have been implicated in different aspects of plant development and plant cell physiology, with much of the supporting data coming from the time- or cell- specific localization of AGPs glycosidic epitopes recognized by several MABs.10 Sporadically, some of these MABs have localized AGPs in the tonoplast or the vacuole.11,12 In C. roseus leaves, the AGP epitope recognized by the MAB Jim13 localizes at the inner face of the tonoplast of many of the mesophyll cells (Fig. 2B), with a punctuated distribution which mirrors the localization pattern of CrPrx1 (Fig. 2A). Moreover, recent proteomic studies have detected the presence of AGPs, Prxs and NADPH oxidase in lipid rafts of certain tissues,13–15 this latter having also been detected in the tonoplast/vacuole.16 In view of all this data, we hypothesize that Prxs may co-localize with AGPs in lipid rafts in both the plasma membrane and the tonoplast, and that the specific localization/orientation of Prxs on the rafts will determine which compounds will function as Prx substrates, namely due to co-localization with specific transporters and with an NADPH oxidase providing the H2O2.
Figure 2.
(A) Cytochemical localization of CrPrx1 in C. roseus mesophyll cells using DAB and H2O2. (B) Green—immunofluorescence localization of AGPs using MAB Jim13 in C. roseus mesophyll cells. Blue—cell walls stained with calcofluor. Red—chloroplast autofluorescence. Bar = 4 µm.
There are thus exciting and challenging questions ahead! Does the tonoplast also present AGPs and lipid rafts? Are Prxs and AGPs indeed associated to lipid rafts? Does this lead to metabolic channeling involving transporters of secondary metabolites? Is there a tonoplast based NADPH oxidase and is this the source of the H2O2 used by Prxs?
Abbreviations
- AGP
arabinogalactan protein
- AVLB
α-3∼,4∼-anhydrovinblastine
- CrPrx1
Catharanthus roseus peroxidase 1
- Prx
class III peroxidase
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6576
References
- 1.Endo T, Goodbody A, Vukovic J, Misawa M. Enzymes from Catharanthus roseus cell suspension cultures that couple vindoline and catharanthine to form 3∼,4∼-anhydrovinblastine. Phytochemistry. 1988;27:2147–2149. [Google Scholar]
- 2.Misawa M, Endo T, Goodbody A, Vukovic J, Chapple C, Choi L, Kutney JP. Synthesis of dimeric indole alkaloids by cell free extracts from cell suspension cultures of Catharanthus roseus. Phytochemistry. 1988;27:1355–1359. [Google Scholar]
- 3.Smith JI, Amouzou E, Yamagushi A, McLean S, DiCosmo F. Peroxidase from bioreactor-cultivated Catharanthus roseus cell cultures mediates biosynthesis of α-3∼,4∼-anhydrovinblastine. Biotechnol Appl Bioeng. 1988;10:568–575. [Google Scholar]
- 4.Sottomayor M, De Pinto MC, Salema R, DiCosmo F, Pedreño MA, Ros Barcelo A. The vacuolar localization of a basic peroxidase isoenzyme responsible for the synthesis of α-3∼,4∼-anhydrovinblastine in Catharanthus roseus (L.) G. Don leaves. Plant Cell Environm. 1996;19:761–767. [Google Scholar]
- 5.Sottomayor M, Lopez-Serrano M, DiCosmo F, Ros Barceló A. Purification and characterization of alpha-3∼,4∼-anhydrovinblastine synthase (peroxidase-like) from Catharanthus roseus (L.) G. Don. FEBS Lett. 1998;428:299–303. doi: 10.1016/s0014-5793(98)00551-1. [DOI] [PubMed] [Google Scholar]
- 6.Sottomayor M, Ros Barceló A. Peroxidase from Catharanthus roseus (L.) G. Don and the biosynthesis of alpha-3∼,4∼-anhydrovinblastine: a specific role for a multifunctional enzyme. Protoplasma. 2003;222:97–105. doi: 10.1007/s00709-003-0003-9. [DOI] [PubMed] [Google Scholar]
- 7.Costa MMR, Hilliou F, Duarte P, Pereira LG, Almeida I, Leech M, Memelink J, Barcelo AR, Sottomayor M. Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol. 2008;146:403–417. doi: 10.1104/pp.107.107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sottomayor M, Ros Barceló A. Plant peroxidases and phytochemistry. Phytochem Rev. 2004;3:1–256. [Google Scholar]
- 9.Deus-Neumann B, Zenk MH. A highly selective alkaloid uptake system in vacuoles of higher plants. Planta. 1984;162:250–260. doi: 10.1007/BF00397447. [DOI] [PubMed] [Google Scholar]
- 10.Seifert GJ, Roberts K. The biology of arabinogalactan proteins. Annu Rev Plant Biol. 2007:137–161. doi: 10.1146/annurev.arplant.58.032806.103801. [DOI] [PubMed] [Google Scholar]
- 11.Pennell RI, Knox JP, Scofield GN, Selvendran RR, Roberts K. A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J Cell Biol. 1989;108:1967–1977. doi: 10.1083/jcb.108.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samaj J, Samajova O, Peters M, Baluska F, Lichtscheidl I, Knox JP, Volkmann D. Immunolocalization of LM2 arabinogalactan protein epitope associated with endomembranes of plant cells. Protoplasma. 2000;212:186–196. [Google Scholar]
- 13.Borner GHH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, MacAskill A, Napier JA, Beale MH, Lilley KS, Dupree P. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 2005;137:104–116. doi: 10.1104/pp.104.053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefebvre B, Furt F, Hartmann MA, Michaelson LV, Carde JP, Sargueil-Boiron F, Rossignol M, Napier JA, Cullimore J, Bessoule JJ, Mongrand S. Characterization of lipid rafts from Medicago truncatula root plasma membranes: A proteomic study reveals the presence of a raft-associated redox system. Plant Physiol. 2007;144:402–418. doi: 10.1104/pp.106.094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mongrand S, Morel J, Laroche J, Claverol S, Carde JP, Hartmann MA, Bonneu M, Simon-Plas F, Lessire R, Bessoule JJ. Lipid rafts in higher plant cells: Purification and characterization of triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem. 2004;279:36277–36286. doi: 10.1074/jbc.M403440200. [DOI] [PubMed] [Google Scholar]
- 16.Carter C, Pan SQ, Jan ZH, Avila EL, Girke T, Raikhel NV. The vegetative vacuole proteorne of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell. 2004;16:3285–3303. doi: 10.1105/tpc.104.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]