Abstract
The INHIBITOR OF MERISTEM ACTIVITY (IMA) gene from tomato regulates the processes of flower and ovule development. 1 IMA encodes a Mini Zinc Finger (MIF) protein that is characterized by a very short sequence containing an unusual zinc-finger domain. IMA acts as a repressor of WUSCHEL expression which controls the meristem organizing centre and the determinacy of the nucellus during ovule development. IMA inhibits cell proliferation during floral termination, controls the number of carpels during floral development and participates in the initiation of ovule primordia by activating D-type gene expression. In addition IMA is involved in a multiple hormonal signalling pathway like its Arabidopsis homolog MIF1.2 We thus propose that IMA, as a representative of this new family of zinc finger proteins, is an important effector in the regulatory pathway controlling meristem activity linking cell division, differentiation and hormonal control of development.
Key words: FM termination, flower development, hormone signalisation, IMA, meristem activity, MIF protein, ovule development
Meristems at both ends of the plant body are responsible for continuous plant development and growth. All aerial parts of the plant originate from the activity of the shoot apical meristem (SAM) which upon floral induction turns into inflorescence meristem and subsequently into floral meristem (FM). Unlike the SAM, the FM displays a determinate growth leading to the formation of a limited number of organs after cessation of meristematic activity and proper differentiation of the ovary including all floral pieces, and the gametophyte. This process called floral termination is under the control of a negative feedback loop between the stem cell promoting gene WUSCHEL (WUS) and the stamen- and carpel-identity gene AGAMOUS (AG).3,4 After floral induction the floral identity gene LEAFY (LFY) and WUS are expressed and activate AG in the centre of the FM which in turn represses WUS to trigger properly the floral termination.
The meristem activity is intimately linked to hormonal growth factors such as cytokinins (CK), auxins and gibberellins (GA) that stimulate cell proliferation and cell elongation. However little is known so far about the integration of multi-hormonal signals, especially during flower induction and floral development.
The proteins belonging to the MIni Zinc Finger (MIF) family have been almost recently characterized in Arabidopsis.2 These proteins display a very small primary sequence (∼100 amino acids) and harbour a unique structural domain made of a putative zinc finger of the CX3HX11CX12–26CX2CXCHX3H type, firstly identified in plant-specific zinc finger homeodomain (ZF-HD) proteins from in vitro analysis of plant DNA-binding proteins.5 In Arabidopsis, the MIF protein family encompasses three members (MIF1 to MIF3). MIF1 as a representative member of this protein family was shown to be involved in multiple hormonal regulations during Arabidopsis development, acting as a plant growth inhibitor.2 However, the precise developmental programs that MIF1 could regulate still wait for elucidation.
From a differential hybridization screen aimed at isolating genes preferentially expressed at the cell division phase of early fruit development,6 we have been able to isolate the IMA gene as the tomato homolog (MIF2-like) of the Arabidopsis MIF2 gene. IMA is indeed preferentially expressed during reproductive (flower and fruit) development in tomato, while the expression of the tomato MIF1-like gene is restricted to vegetative organs. The novelty of our work was to provide the first description of loss-of-function transgenic lines for such a gene, thus making it possible to target developmental programs involving a MIF2-like gene such as IMA.
IMA, a General Plant Growth Inhibitor
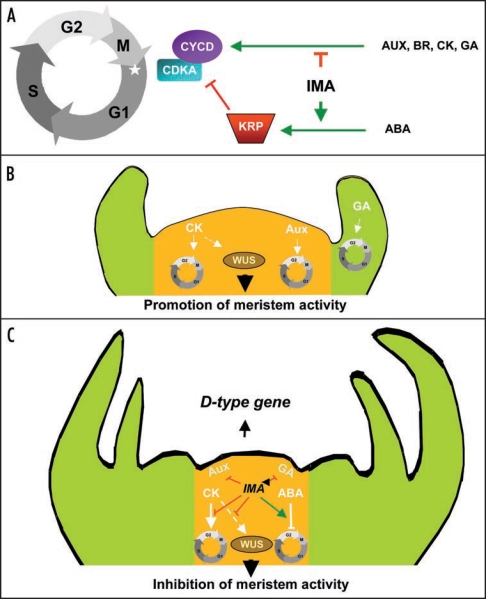
Alike MIF1 and MIF2 genes in Arabidopsis,2 IMA has a clear inhibiting effect on plant growth in tomato. Indeed, overexpressing IMA leads to a slowdown and a decline in plant growth through the alteration of cell divisions. Most probably through an indirect way, IMA could influence the expression of cell cycle regulatory genes. Indeed, the level of expression of genes involved in the commitment to cell cycle such as the G1/S-specific cyclin gene CYCD3;1,7 and the commitment to mitosis such as the G2/M specific Cyclin Dependent Kinase gene CDKB2;1,7 are inversely correlated with the level of IMA transcripts. In addition the transcripts for KRP1, a specific inhibitor of CDK-Cyclin complexes8 strongly accumulated in IMA overexpressors. Interestingly we have been able to demonstrate that IMA influences negatively the response to growth factors, such as auxin, cytokinin, brassinosteroids and gibberellins, known to activate the transcription of D-type cyclins for entry into the cell cycle.7 Conversely IMA has a positive effect on antimitogenic stimuli, such as abscissic acid which activates the expression of CDK/Cyclin complex inhibitors.9 IMA seems to be involved in the control of plant growth at the crossroads of hormonal signalling pathways and cell cycle regulation, by preventing cells from entering into proliferation (Fig. 1A).
Figure 1.
Tentative model for the function of IMA in the regulation of meristematic activity and flower determination. (A) IMA as a general inhibitor of plant growth linking hormonal perception and cell cycle control. (B) Promotion of meristem activity in the absence of IMA gene expression. (C) Inhibition of meristem activity by IMA. IMA prevents the perception of growth stimulatory hormonal signals, represses the expression of WUSCHEL and promotes D-type gene expression.
IMA, a “Switch” for Meristematic Activity During Floral Development
The study of spatial and temporal expression of IMA and its functional analysis demonstrated the involvement of IMA in the control of floral meristematic activity through its inhibitory function.1 Plant meristem activity is linked to the control and maintenance of cell divisions which are intimately connected to hormonal control. Auxin and gibberellins induce the formation of lateral organ primordia through the activation of periclinal cell divisions, while the maintenance of the meristem activity is highly dependent on cytokinins that affect directly the expression of WUS,10 (Fig. 1B). We have been able to show that IMA represses WUS expression thus leading to FM termination. In addition, since IMA is involved in the impairment of auxin, gibberellin and cytokinin signalling perception, it must participate in the disappearance of the meristematic activity in the centre of the flower (Fig. 1C). Moreover, IMA must also contribute to the increase of cell sensitivity to ABA, thus reducing the perception of cytokinin signalling and consequently the cell division activity within the FM.
The repression of WUS during the FM termination is thought to be mediated by transcription factor complexes consisting in C-, D- and E-function MADS-box proteins.11 IMA was shown to activate D-function genes and therefore IMA could constitute an intermediary between the C- and D-functions, allowing the formation of C/D/E transcriptional complex known as MADS-box quartet,12 through the activation of D-function genes, and thus the inhibition of the meristematic activity (Fig. 1C).
IMA Participates in the Ovule Identity
The most spectacular phenotypes obtained during the functional analyses of IMA in tomato are related to ovule development.1 Indeed the upregulation of IMA increased the number of ovules as a result of its positive regulation on the expression of the D-function gene TAgl11 which specifies ovule identity.11 Conversely the downregulation of IMA resulted in the downregulation of TAgl11 and a dramatic impairment in the differentiation of both ovule and nucellus giving rise to undifferentiated finger-like structures primarily comprised of integument tissue and devoid of embryo sac.1 These undifferentiated structures are the result of a maintained cell proliferation which is defined molecularly by the sustained WUS expression, in accordance with the previously reported function of WUS during integument and nucellus development.13 These data therefore indicate that the shift from the indeterminate state to the determinate state and then to nucellus differentiation is intimately linked to the repression of meristem-associated genes during ovule development, as a result of WUS inhibition within the nucellus primordium, under the control of IMA.
Conclusion and Perspectives
Our work in tomato demonstrated the importance of the IMA (MIF2-like) gene during flower and ovule development. The precise mode of molecular action of MIF proteins is still unknown. These proteins cannot be defined as transcription factors per se as they do not bind to DNA,2,5 but most probably they act through protein-protein interactions mediated by the zinc finger domain, for instance by sequestering other proteins such as transcription factors and preventing them to act on their cis-element targets. Currently an interactome approach is underway as to decipher the putative protein partners of IMA and its role in the regulatory pathway controlling meristem activity.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6647
References
- 1.Sicard A, Petit J, Mouras A, Chevalier C, Hernould M. Meristem activity during flower and ovule development is controlled by the mini zinc finger gene INHIBITOR OF MERISTEM ACTIVITY in tomato. Plant J. 2008;55:415–427. doi: 10.1111/j.1365-313X.2008.03520.x. [DOI] [PubMed] [Google Scholar]
- 2.Hu W, Ma H. Characterization of a novel putative zinc finger gene MIF1: involvement in multiple hormonal regulation of Arabidopsis development. Plant J. 2006;45:399–422. doi: 10.1111/j.1365-313X.2005.02626.x. [DOI] [PubMed] [Google Scholar]
- 3.Lenhard M, Bohnert A, Jürgens G, Laux T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell. 2001;105:805–814. doi: 10.1016/s0092-8674(01)00390-7. [DOI] [PubMed] [Google Scholar]
- 4.Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell. 2001;105:793–803. doi: 10.1016/s0092-8674(01)00384-1. [DOI] [PubMed] [Google Scholar]
- 5.Windhövel A, Hein I, Dabrowa R, Stockhaus J. Characterization of a novel class of plant homeodomain proteins that bind to the C4 phosphoenolpyruvate carboxylase gene of Flaveria trinervia. Plant Mol Biol. 2000;45:201–214. doi: 10.1023/a:1006450005648. [DOI] [PubMed] [Google Scholar]
- 6.Joubes J, Phan TH, Just D, Rothan C, Bergounioux C, Raymond P, Chevalier C. Molecular and biochemical characterization of the involvement of cyclin-dependent kinase A during the early development of tomato fruit. Plant Physiol. 1999;121:857–869. doi: 10.1104/pp.121.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inzé D, De Veylder L. Cell cycle regulation in plant development. Annu Rev Genet. 2006;40:77–105. doi: 10.1146/annurev.genet.40.110405.090431. [DOI] [PubMed] [Google Scholar]
- 8.Bisbis B, Delmas F, Joubès J, Sicard A, Hernould M, Inzé D, Mouras A, Chevalier C. Cyclin-Dependent Kinase Inhibitors are involved in endoreduplication during tomato fruit development. J Biol Chem. 2006;281:7374–7383. doi: 10.1074/jbc.M506587200. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke LC. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J. 1998;15:501–510. doi: 10.1046/j.1365-313x.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- 10.Leibfried A, To JPC, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438:1172–1175. doi: 10.1038/nature04270. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario S, Shchennikova AV, Franken J, Immink RGH, Angenent GC. Control of floral meristem determinacy in Petunia by MADS-Box transcription factors1. Plant Physiol. 2006;140:890–898. doi: 10.1104/pp.105.072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theissen G, Saedler H. Floral quartets. Nature. 2001;409:469–471. doi: 10.1038/35054172. [DOI] [PubMed] [Google Scholar]
- 13.Gross-Hardt R, Lenhard M, Laux T. WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes Dev. 2002;16:1129–1138. doi: 10.1101/gad.225202. [DOI] [PMC free article] [PubMed] [Google Scholar]