Abstract
Developmental plasticity defines an adaptive mechanism, which plays a fundamental role in plant development and survival. How intrinsic or extrinsic factors are integrated to specify cell fates and subsequent organ and body building of a plant is still poorly understood. By studying developmental plasticity of Arabidopsis root hair in response to salt stress, we have begun to understand more about the basis of cellular plasticity. This paper summarizes our recent paper in which it described salt stress induced plasticity of root epidermis and root hair development in Arabidopsis. Analysis of gene expression of the homeobox transcription factor GLABRA2 (GL2), which determines hair/non-hair cell fate, showed that salt stress modulates root epidermal cell proliferation and changes the cell fate decisions. Furthermore, by analyzing the salt overly sensitive (sos) mutants, we showed that salt-induced root hair plastic response is caused by ion disequilibrium and it appears to be adaptive mechanism. Based on the most recent discoveries, we propose here that chromatin remodeling and epigenetic control may be the basis for cell fate changes and the ultimately adaptive plasticity in response to transient changes of environmental conditions.
Key words: adaptation, Arabidopsis, cell fate, chromatin remodeling, developmental plasticity, root hair, salt stress
Plants, as sessile organisms, have to get along with the dynamics of transiently changing environmental conditions during their lifetime.1–3 Developmental plasticity is essential for stress adaptation in plants. Therefore, the basis of adaptation or cellular plasticity to varying environmental conditions is the central question of plant developmental biology. However, how plant cells integrate intrinsic and extrinsic signals to direct cell fates and to achieve unique developmental goals remains largely unknown. A model of choice to study cell fate control and cellular plasticity in plants is the Arabidopsis root epidermis development, because it is easy to study and is very suitable for analysis of cell differentiation and morphorgenesis. In deed, great progresses have been made in understanding the regulation of cell division, patterning and differentiation in Arabidopsis root epidermal cells.4–13
In the Arabidopsis root, meristematic epidermis cells divide into a particular pattern4. The cells locating over the anticlinal wall separating two cortical cells differentiate into cells that normally bear root hairs (trichoblasts), whereas those lied over the outer periclinal cortical cell walls become hairless cells (atrichoblasts).4,5 It is known that root epidermal cell fate decisions are trigged by a positional cue from the underlying cortical cell layer, and cell fate specification and subsequent cell differentiation into trichoblasts and atrichoblasts are regulated by a complex transcription factor network that regulates the expression of GL2. Expression of GL2 then determines hair or non-hair cell fate, and it is only expressed in atrichoblasts.6–8 This particular cell patterning is established during embryogenesis and maintained during postembryonically.9–12 Recent findings also revealed that root epidermal cell division, patterning and differentiation are also modulated through regulation of root epidermis patterning gene expression by histone modification and chromatin reorganization.13–15 Chromatin restructuring at GL2 locus appears to be essential for position-dependent cell fate specification and root epidermal development. Importantly, GL2 expression and cell fate determination are reset in each cell cycle. It is proposed that this regulatory mechanism of cell fate decision may be the molecular basis for cellular plasticity of root epidermis.13–15
Our research examined the developmental plasticity of Arabidopsis root epidermis induced by salt stress.16 We have shown that salt stress markedly influence root epidermis development and subsequent root hair development. Salt stress reduces the number of non-hair cells and disrupts the determined pattern of root epidermis. The normal pattern of root epidermis is composed of two rows of hairless cells and one row of hair cells next to them under normal conditions. However, root epidermis cell pattern become abnormal when exposed to salt stress (100 mM NaCl) consisting of one row hair by one row of non-hair cells. This observation indicates that salt stress changes root epidermal cell proliferation. Interestingly, by analyzing GL2 expression, we found that salt stress alters cell fate specification. Under salt stress, some trichoblasts become atrichoblasts, because GL2 expression was detected in these cells although they are still in the hair cells position. It is apparent that salt stress disturbed the position-dependent expression pattern of GL2 and subsequent cell fate decision. As a result, number of root hairs was substantially reduced by salt stress, but the root hairs initiated in the correct site at apical end of a trichoblast. Furthermore, root hair tip growth was also inhibited by salt stress. Importantly, root epidermis exhibits a rapid reversible cellular plasticity where they can switch their above phenotypes back and forth in response to the changes of salt stress. The disrupted development of root epidermis can be restored during prolonged treatment of low level of salt (25 mM NaCl), indicating that an adaptive response does take place.
Our results showed that cell fate plasticity is crucial for developmental plasticity. The root meristematic epidermal cells retain abilities to reprogram their cell fates in response to changing external stimuli. Under normal conditions, trichoblasts and atrichoblasts in the meristematic epidemics divide transversely to form alternating files of hair and non-hair cells. Trichoblasts then differentiate root hairs, and atrichoblasts bear no hairs. Exposure to salt stress, ecotopic expression of GL2 is activated resulting in the cell fate switch and differentiation of atrichoblast in hair position. On the contrary, we assume that when exposed to nutrient deficiency like phosphorus and iron, GL2 is silenced in atrichoblasts and root hairs form in inappropriate positions (non-hair position). Therefore, low concentrations of available phosphorus and iron in the rhizosphere induce a significant increase in the number of root hairs to facilitate adaptation.17–20 It is likely that root meristematic epidermis cells have capacity to change their determined cell fate by regulating the activity of the homeotic genes, such as GL2, during development in response to varying environmental stimuli. Such developmental plasticity has been observed in other systems, such as Drospohila and mammals.21–24 For example, activation of certain homeotic genes results in cell fate switch and transdetermination of different organs during development, and epigenetic control of chromatin assembly has been proposed to be the basis to establish or to maintain determined states.
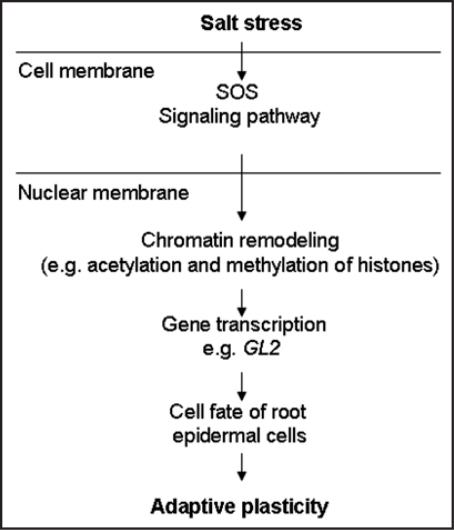
Our results and the recent discoveries support the notion that chromatin remodeling and epigenetic control is the molecular basis of the plasticity in cell fate switch of root epidermis in response to changing environment conditions. In the case of salt stress induced cell fate changes of root epidermis, it is likely that salt stress, when the salt signal is sensed and tranduced through signaling pathways, such as the SOS pathway,25–27 in combination with other cellular factors, influences chromatin states, which in turn alter cell fate specification, cell division and root epidermis patterning in Arabidopsis (Fig. 1). Thus, chromatin remodeling and epigenetic control appears to function as a general developmental tool that is used to direct cell fate and, consequently, to affect organ formation and morphogenesis.14,28,29 The fact that chromatin states and cell fates are reset in each cell cycle during root epidermal development could easily explains why root hair development can rapidly adapt to a transiently changing environment. Thus, capacity of rapid chromatin remodeling and cell fate switch in response to changing environments is necessary for cellular adaptive plasticity to minimize damage or protect themselves from upcoming stress. However, we do not exclude other possibilities in regulating stress induced celluar plasticity of root epidermis. The mechanisms by which salt signal induces chromatin remodeling of root meristematic epidermis cells or by which epigenetic control of specific genes to coordinate adaptive response remains yet to be established. Further research is needed to elucidate the underlying mechanisms of salt induced plasticity of root epidermal development that might lead to a better understanding of adaptations of plants to salinity stress.
Figure 1.
A proposed model that may modulate salt stress induced cellular plasticity of root epidermis patterning in Arabidopsis.
Acknowledgements
This work was supported by One hundred Talents Program of Chinese Academy of Sciences. It also was partially supported by a Chinese NSFC grant (30570143).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5759
References
- 1.Amazallag GN. Maturation of integrated functions during development. I. Modifications of the regulatory network during transition periods in sorghum biocolor. Plant Cell Envion. 2001;24:337–345. [Google Scholar]
- 2.Arnholdt-Schmitt B. Stress-induced cell reprogramming. A role for global genome regulation? Plant Physiol. 2004;136:2579–2586. doi: 10.1104/pp.104.042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller M, Schmidt W. Environmentally induced plasticity of root hair development in Arabidopsis. Plant Physiol. 2004;134:409–419. doi: 10.1104/pp.103.029066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan L, Duckett C, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poething S, Roberts K. Clonal relathionships and cell patterning in the root epidermis of Arabidopsis. Development. 1994;120:2465–2474. [Google Scholar]
- 5.Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166:740–754. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- 6.Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- 7.Di Cristina M, Sessa G, Dolan L, Linstead P, Baima S, Ruberti I, Morelli G. The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. Plant J. 1996;10:393–402. doi: 10.1046/j.1365-313x.1996.10030393.x. [DOI] [PubMed] [Google Scholar]
- 8.Hung CY, Lin Y, Zhang M, Pollock S, Marks MD, Schiefelbein J. A common position- dependent mechanism controls cell-type patterning and GLABRA2 regulation in the root and hypocotyl epidermis of Arabidopsis. Plant Physiol. 1998;117:73–84. doi: 10.1104/pp.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger F, Haseloff J, Schiefellbein J, Dolan L. Positional information in the root epidermis is defeined during embryogenesis and acts in domains with strict boundaries. Curr Biol. 1998;8:421–430. doi: 10.1016/s0960-9822(98)70176-9. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y, Schiefellbein J. Embryonic control of wpidermal cell patterning in the root and hypocotyl of Arabidopsis. Development. 2001;128:3697–3705. doi: 10.1242/dev.128.19.3697. [DOI] [PubMed] [Google Scholar]
- 11.Costa S, Dolan L. Epidermal patterning genes are active during embryogenesis in Arabidopsis. Development. 2003;130:2839–2901. doi: 10.1242/dev.00493. [DOI] [PubMed] [Google Scholar]
- 12.Kwak SH, Shen R, Schiefellbein J. Positional signaling mediated by a receptor-like kinase in Arabidopsis. Science. 2005;307:1111–1113. doi: 10.1126/science.1105373. [DOI] [PubMed] [Google Scholar]
- 13.Costa S, Shaw P. Chromatin organization and cell fate switch respond to positional information in Arabidopsis. Nature. 2006;439:493–496. doi: 10.1038/nature04269. [DOI] [PubMed] [Google Scholar]
- 14.Costa S, Shaw P. ‘Open minded’ cells: how cells can change fate. Trends Cell Biol. 2006;17:101–106. doi: 10.1016/j.tcb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Caro E, Castellano MM, Gutierrez C. A chromatin link that couples cell division to root epidermis patterning in Arabidopsis. Nature. 2007;447:213–217. doi: 10.1038/nature05763. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Zhang W, Li K, Sun F, Han C, Wang Y, Li X. Salt-induced plasticity of root hair development is caused by ion disequilibrium in Arabidopsis thaliana. J Plant Res. 2008;121:87–96. doi: 10.1007/s10265-007-0123-y. [DOI] [PubMed] [Google Scholar]
- 17.Schikora A, Schmidt W. Iron stress-induced changes in root epidermal cell fate are regulated independently from physiological responses to low iron availability. Plant Physiol. 2001;125:1679–1687. doi: 10.1104/pp.125.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilroy S, Jones DL. Through form to function: root hair development and nutrient uptake. Trends Plant Sci. 2000;5:56–60. doi: 10.1016/s1360-1385(99)01551-4. [DOI] [PubMed] [Google Scholar]
- 19.Ma Z, Bielenberg DG, Brown KM, Lynch JP. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 2001;24:459–467. [Google Scholar]
- 20.Schmidt W, Tittel J, Schikora A. Role of hormone in the induction of Fe deficiency responses in Arabidopsis roots. Plant Physiol. 2000;122:1109–1118. doi: 10.1104/pp.122.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maves L, Schubige G. Transdetermination in Drosophila imaginal discs: a model for understanding pluripotency and selector gene maintenance. Curr Opin Genet Dev. 2003;13:472–479. doi: 10.1016/j.gde.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Wei G, Schubige G, Harder F, Muller AM. Stem cell plasticity in mammals and trans-determination in Drosophila: common themes? Stem Cells. 2000;18:409–414. doi: 10.1634/stemcells.18-6-409. [DOI] [PubMed] [Google Scholar]
- 23.Maurange C, Lee N, Paro R. Signaling meets chromatin during tissue regeneration in Drosophila. Curr Opin Genet Dev. 2006;16:485–489. doi: 10.1016/j.gde.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Gibert J, Peronnet F, Schlötterer Phenotypic plasticity in Drosophila pigmentation caused by temperature sensitivity of a chromatin regulator network. PLoS Genet. 2007;3:30. doi: 10.1371/journal.pgen.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu JK, Liu J, Xiong L. Genetic analysis of salt tolerance in Arabidopsis: evidence for a role of potassium nutrition. Plant Cell. 1998;10:1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu JK. Salt and drought stress signal transduction in plants. Ann Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin in Plant Biol. 2003;6:441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 28.Molinier J, Ries G, Zipfel C, Hohn B. Transgeneration memory of stress in plants. Nature. 2006;442:1046–1049. doi: 10.1038/nature05022. [DOI] [PubMed] [Google Scholar]
- 29.Gendall AR, Levy YY, Wilson A, Dean C. The VERNALIZATION2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell. 2001;107:525–535. doi: 10.1016/s0092-8674(01)00573-6. [DOI] [PubMed] [Google Scholar]