Abstract
Starch branching enzyme (SBE) activity in the cassava storage root exhibited a diurnal fluctuation, dictated by a transcriptional oscillation of the corresponding SBE genes. The peak of SBE activity coincided with the onset of sucrose accumulation in the storage, and we conclude that the oscillatory mechanism keeps the starch synthetic apparatus in the storage root sink in tune with the flux of sucrose from the photosynthetic source. When storage roots were uncoupled from the source, SBE expression could be effectively induced by exogenous sucrose. Turanose, a sucrose isomer that cannot be metabolized by plants, mimicked the effect of sucrose, demonstrating that downstream metabolism of sucrose was not necessary for signal transmission. Also glucose and glucose-1-P induced SBE expression. Interestingly, induction by sucrose, turanose and glucose but not glucose-1-P sustained an overt semidian (12-h) oscillation in SBE expression and was sensitive to the hexokinase (HXK) inhibitor glucosamine. These results suggest a pivotal regulatory role for HXK during starch synthesis. Abscisic acid (ABA) was another potent inducer of SBE expression. Induction by ABA was similar to that of glucose-1-P in that it bypassed the semidian oscillator. Both the sugar and ABA signaling cascades were disrupted by okadaic acid, a protein phosphatase inhibitor. Based on these findings, we propose a model for sugar signaling in regulation of starch synthesis in the cassava storage root.
Key words: cassava, SBE, semidian oscillation, starch synthesis, sugar signaling
Introduction
In plants, sugar sensing and signaling play critical roles in controlling many aspects of growth, metabolism and development throughout the whole plant life cycle.1–4 Through photosynthesis, plants convert atmospheric carbon dioxide to sugars, which are transported as sucrose from sugar-exporting (source) organs, such as leaves, to sugar-importing (sink) organs, such as tubers, seeds or storage roots. The coordinated modulation of gene expression in source and sink organs is to a large extent choreographed by the sugar status in the cells (reviewed in ref. 5 and refs. therein). In general, low sugar levels promote photosynthesis and mobilization of energy reserves, whereas high sugar levels stimulate growth and storage of starch and other carbohydrates.
Development of sink organs is orchestrated by the coordinated activities of a large number of genes that encode metabolic and regulatory enzymes, as well as other proteins. Starch synthesis is catalyzed by an enzymatic machinery containing ADP-glucose pyrophosphorylase (AGPase), starch synthases (SS), starch branching enzymes (SBE), and starch debranching enzymes (DBE). Most, if not all, of these enzymes exist in two or more isoforms (reviewed in refs. 6–8 for reviews on starch biosynthesis).
Cassava (Manihot esculenta Crantz L.) is a perennial starch crop of major global importance, particularly in the Developing World.9,10 Starch is synthesized and deposited in the underground tuberous storage roots, which can measure up to 1 m in length and over 10 cm in diameter. The storage root is a non-reproductive organ and can accumulate close to 85% of its total dry weight as starch.
During development of the cassava storage root, expression of the SBEII and SBEI genes, encoding, respectively, SBEII and SBEI, gradually increases from 90 to 360 days after planting (d.a.p.).11 Accumulation of SBEII and SBEI transcripts in 360 d.a.p. plants was also found to exhibit a short-term fluctuation with a period of one day. In the work presented here, we followed the induction of SBE expression in isolated cassava storage root discs by sugars, sugar analogs and abscisic acid (ABA). Our results establish the presence of an endogenous semidian (12-h) oscillator in the storage root cells at the level of hexokinase (HXK). They also suggest that sugar and ABA signaling proceeds via independent pathways, and that the sugar signaling cascade is activated by the entry of sucrose into the cell and relies on HXK activity.
Results
Diurnal control of SBE expression in the cassava storage root is mediated by a local, semidian oscillator.
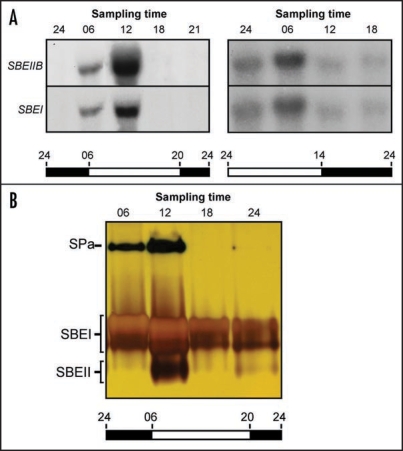
Typical 24 h expression cycles for the cassava SBEII and SBEI genes in the underground storage root from plants grown under two different day-night (LD) regimes are displayed in Figure 1A. Under normal growth conditions, transcription peaked at midday, corresponding to six hours into the light zone. When the onset of light was moved forward, the peak of transcription shifted accordingly. Zymogram analysis revealed that the enzymatic activity of the two proteins fluctuated in accordance with SBEII and SBEI transcript levels (Fig. 1B). This implies that the diurnal rhythm in SBEII and SBEI expression is a regulatory phenomenon and suggests a dynamic behavior of starch synthesis in the storage root during a 24 h period. In addition to the SBEII and SBEI activities, a third activity band was visible on the zymogram. This likely corresponds to endogenous starch phosphorylase a (Sun et al., 2005).
Figure 1.
Activity rhythms in the cassava storage root from plants grown under LD conditions. (A) RNA gel blot analysis. Storage root RNA was extracted from plants grown under two different LD regimens at indicated times during the day and assayed for SBEII and SBEI gene activity. (B) Zymogram analysis. Protein was extracted from storage roots at indicated times during the day and analyzed for starch branching enzyme activity. Times are indicated as follows: 24, midnight; 06, 6 am; 12, noon; 18, 6 pm; 21, 9 pm. SPa, starch phosphorylase a.
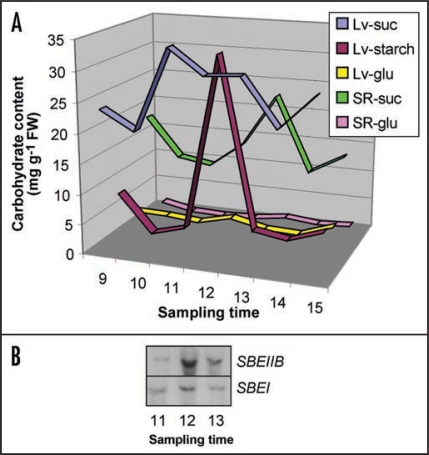
There is ample evidence that sucrose stimulates expression of starch synthesis genes in many plants via a sugar-signaling pathway.2–5,14–16 Thus a likely scenario is that the oscillation in SBEII and SBEI activity in the cassava storage root is controlled by the import of photosynthate, i.e., sucrose, from the source tissues to the underground sink. To probe this hypothesis, we monitored the time course for sucrose accumulation in the cassava storage roots from LD plants (Fig. 2). The results revealed that the onset of sucrose accumulation in the storage root coincided with the maximum levels of SBEII and SBEI transcripts, and that the maximum sucrose concentration was reached at 1 p.m., one hour after the SBEII and SBEI peak time. We interpret these findings to mean that the transcriptional machinery for the SBEII and SBEI genes in the cassava storage root is set to anticipate the incoming sucrose, i.e., the substrate for starch synthesis, from the source. The situation becomes analogous to the circadian rhythm of photosynthesis genes, which prepares the pre-dawn photosynthetic cell for the approaching light exposure.
Figure 2.
Changes in carbohydrate levels in the cassava storage root from plants grown under LD conditions. (A) Starch and sugars were extracted at indicated times during the day. Lv-suc, leaf sucrose; Lv-starch, leaf starch; Lv-glu, leaf glucose; SR-suc, storage root sucrose; SR-glu, storage root glucose. (B) SBEII and SBEI gene activity between 11 am (11) and 1 pm (13). Times are indicated as follows: 9, 9 am; 10, 10 am; 11, 11 am; 12, noon; 13, 1 pm; 14, 2 pm; 15, 3 pm.
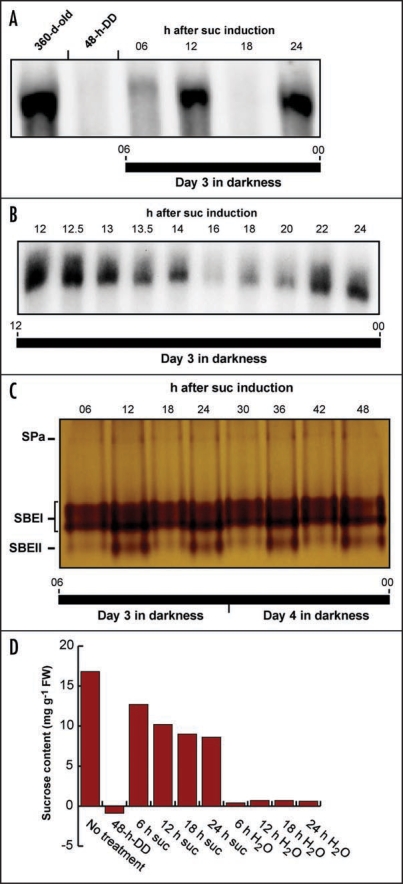
To further our understanding of the temporal influence of sucrose on SBE expression in the storage roots, we uncoupled the sink organs from source control and examined SBEII expression in cassava root discs from plants that had been kept in continuous darkness (DD) for 48 h to remove endogenous metabolizable sugars. The root slices were maintained under DD conditions to mimic the natural environment and transferred to sucrose medium. No SBEII expression could be detected in discs on non-sucrose medium (Fig. 3A). Shifting to sucrose-containing medium efficiently increased endogenous sucrose levels and SBEII transcripts appeared within two hours. Surprisingly, both transcription and enzyme activity in the isolated root discs oscillated with a period of approximately 12 h (Fig. 3B and C). Since the sucrose concentrations did not change accordingly but, rather, showed a slight decline (Fig. 3D), the results clearly demonstrate that the observed oscillations are not governed by fluctuations in the sucrose output form the source tissues but originate from an endogenous, semidian (12-h) oscillator in the storage root itself. The oscillations persisted without any damping beyond day 2 after transfer to sucrose medium.
Figure 3.
Sucrose induction of SBE expression and SBE activity in isolated discs of cassava storage roots kept under DD conditions. (A) RNA gel blot analysis of SBE expression. Root discs from dark-adapted plants (360-d-old) were depleted of endogenous sugars for 48 h (48-h-DD) and then transferred to sucrose medium. RNA was extracted at indicated times after sugar induction and assayed for SBEII and SBEI activity. (B) RNA gel blot analysis of SBE expression. A detailed time-course for SBEII and SBEI gene activity in the interval 12–24 h after sugar induction. (C) Zymogram analysis. Protein was extracted from storage root discs at indicated times after sugar induction and analyzed for starch branching enzyme activity. (For identification of the activity bands, see Sun et al., 2005). (D) Sucrose content. Sugars were extracted from storage root discs depleted of endogenous sugars for 48 h (48-h-DD) and at indicated times after addition of sucrose or H2O. SPa, starch phosphorylase a, Suc, sucrose.
Sugar-induced semidian oscillation of SBE expression relies on sucrose sensing at the plasma membrane and HXK activity.
Employing different sugars and sugar analogs potentially can yield valuable clues regarding the mechanisms of the sugar-signaling pathway. This is particularly true since exogenously supplied sucrose can readily convert to hexoses in the apoplast or cytosol, obscuring interpretations as to whether the sensed sugar is sucrose or some downstream metabolite. Palatinose (α-glucose-1,6-β-fructose) and turanose (α-glucose-1,3-β-fructose) are both isomers of sucrose (α-glucose-1,2-β-fructose). From the plants so far studied, it is generally considered that palatinose and turanose can neither be recognized by plant sucrose transporters (SUTs), nor be metabolized by plant enzymes.17–20 However, although they are not taken up by plant cells, work on barley embryos,21 potato tubers17 and tobacco leaves22,23 shows that palatinose and turanose can modulate sugar signaling, and it was suggested that they are perceived extracellularly, possibly at the plasma membrane, by disaccharide sensors distinct from SUTs.18,21 Also, at least in one case, for the main phloem-loading SUT in Arabidopsis (AtSUC2), it was shown that turanose could serve as a transported SUT substrate.24 Uptake of turanose was also reported for leaves of garden cress25 although it is not clear if a SUT or some other transporter was engaged.
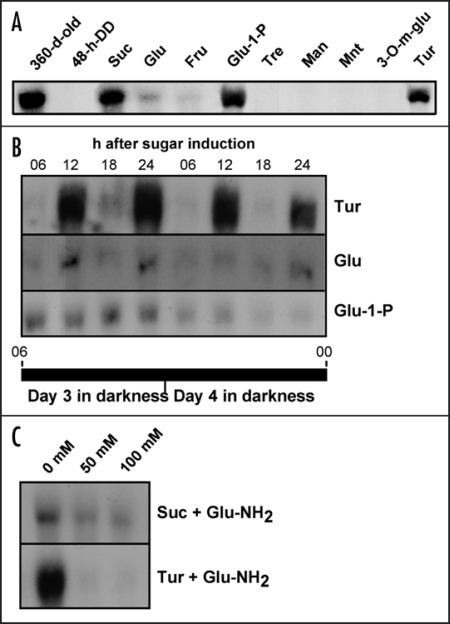
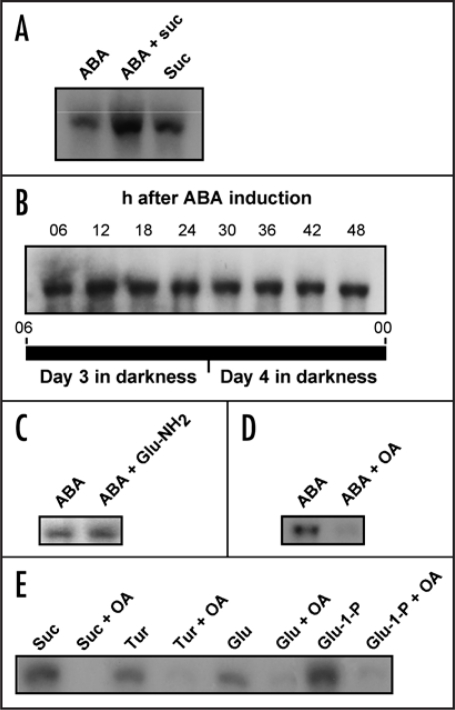
As is demonstrated in Figure 4, addition of turanose to cassava storage roots induced SBE expression to almost the same extent as sucrose. Remarkably, turanose also mimicked sucrose by sustaining the semidian oscillation of SBE transcript levels (Fig. 4B). Glucose and fructose also stimulated SBE expression but were weaker inducers than sucrose. Mannose, which is taken up by the cells and phosphorylated by HXK,18,26,27 as well as mannitol and 3-O-methyl-glucose, which are taken up by the cells but are poor substrates for HXK, had no effect. Also trehalose, which has been implicated in plant sugar signaling,2,4 had no effect.
Figure 4.
RNA gel blot analyses of SBE expression after application of sugars or sugar analogs to isolated discs of cassava storage roots kept under DD conditions. (A) SBE expression after addition of sucrose (Suc), glucose (Glu), fructose (Fru), glucose-1-P (Glu-1-P), trehalose (Tre), mannose (Man), mannitol (Mnt), 3-O-methyl glucose (3-O-m-glu) or turanose (Tur). (B) SBE expression at different time points after addition of turanose, glucose or glucose-1-P (Glu-1-P). Times are indicated as in Figure 1. (C) Sucrose or turanose-induced SBE expression in the presence of 0–100 mM glucosamine (Glu-NH2).
The inability of mannose to induce SBE expression implies that HXK-dependent phosphorylation of an incoming substrate does not suffice to initiate a signaling event, contrary to the phosphotransferase system in bacteria. This by itself, however, does not disqualify HXK as an important element in the intracellular signaling pathway. A possible path would be that the signal initiated at the level of a SUT, or a designated sucrose sensor, in the plasma membrane results in activation of HXK, with subsequent phosphorylation of endogenous glucose. In the intact plant, glucose will be plentiful in developing storage roots but in our experimental system it would likely be derived from mobilization of starch reserves in the amyloplast. To assess the requirement for HXK in sucrose induction of SBE expression, we investigated the effects of sucrose and turanose in the presence of glucosamine, a well-documented HXK inhibitor.2,4,18 The results revealed that induction by turanose was completely abolished and that of sucrose severely diminished after addition of the inhibitor (Fig. 4C). Thus the signal that emanates from sucrose sensing at the plasma membrane depends on HXK activity for downstream transduction.
The finding that the sugar-signaling pathway was disrupted by HXK inhibition, suggested that hexose phosphates, such as glucose-1-P or glucose-6-P, might be potent inducers of SBE expression. To our knowledge, plant plasma membranes do not contain hexose phosphate translocators and, therefore, delivery of extracellular hexose phosphates to storage root slices is expected to be poor. However, we found that the effect of exogenous glucose-1-P on SBE induction was stronger than for glucose and comparable to that of sucrose (Fig. 4A). If the efficient uptake of glucose-1-P points to a plasma membrane glucose transporter with broad substrate specificity, or to some other facilitated transport mechanism is not known. Glucose-1-P and glucose-6-P are equilibrated in the cell by the action of phosphoglucomutase, and so we do not know which of them is the signaling agent, or if they both are equally effective. A noteworthy difference between glucose and glucose-1-P was that glucose, just like sucrose and turanose, supported oscillation of SBE expression, whereas glucose-1-P did not (Fig. 4B).
Based on these findings, we arrive at the following conclusions concerning the sugar-signaling cascade that regulates the semidian SBE expression in the cassava sink cells:
The main trigger for the signaling event operates at the level of sucrose perception or transport, i.e., the arriving sucrose molecule from the source does not need to be hydrolyzed by cell wall invertase or cytosolic enzymes.
HXK activity is crucial for the intracellular signaling pathway, and it is the hexose phosphates, not the phosphorylation step, that mediate the signal.
HXK is activated by sucrose and, possibly, glucose transport at the plasma membrane.
The oscillator that brings about the semidian rhythmicity of SBE expression is located upstream of glucose-1-P/glucose-6-P but downstream of glucose, i.e., at the level of HXK.
ABA promotes induction of SBE expression but bypasses the oscillator.
Sugar signaling does not operate in splendid isolation but rather is integrated in cellular regulatory networks. Most notably, there is a tight interaction between sugar and hormonal signaling, particularly for ABA.2,4,28–30 Work on Arabidopsis has shown that ABA stimulates the accumulation of storage reserves, such as starch,28 and from studies on Arabidopsis leaves it was reported that ABA enhances sucrose-induction of genes encoding SBE2.2 and the small subunit of AGPase.28,31
Addition of ABA to cassava storage root discs effectively induced SBE expression (Fig. 5A). Induction occurred both in the absence or presence of added sucrose, and the effects were additive. Importantly, ABA-induced SBE expression in cassava sink cells was constitutive and did not oscillate (Fig. 5B). Thus induction of SBE expression by both ABA and glucose 1-P bypasses the semidian oscillator. Taken together, these results also tentatively place the influence of ABA in the sugar-hormonal regulatory network downstream of HXK, a notion supported by the insensitivity of ABA induction to glucosamine (Fig. 5C).
Figure 5.
RNA gel blot analyses of SBE expression after application of sucrose, glucose, turanose and/or abscisic acid (ABA) to isolated discs of cassava storage roots kept under DD conditions. (A) Induction of SBE expression by ABA, sucrose or ABA + sucrose. (B) SBE expression at indicated times after induction with ABA. (C) Induction of SBE expression by ABA in the presence or absence of 100 mM glucosamine (Glu-NH2). (D) Induction of SBE expression by ABA in the presence or absence of okadaic acid (OA). (E) Induction of SBE expression by sucrose, turanose, glucose or glucose-1-P (Glu-1-P) in the presence or absence of okadaic acid.
Not surprisingly, several studies have provided evidence for the involvement of reversible phosphorylation events in ABA signaling.14 Two of the genes implicated in ABA signaling turned out to encode protein phosphatases32 and inhibition of protein phosphatase 1 and 2A by okadaic acid was found to alter ABA-induced gene expression.33 As is shown in Figure 5D, okadaic acid inhibited ABA induction of SBE expression. Furthermore, okadaic acid inhibited also the induction by sucrose, turanose, glucose and glucose-1-P (Fig. 5E), suggesting that protein phosphatase 1 or 2A might be a common regulator for the sugar and ABA signaling pathways in the cassava storage root.
Discussion
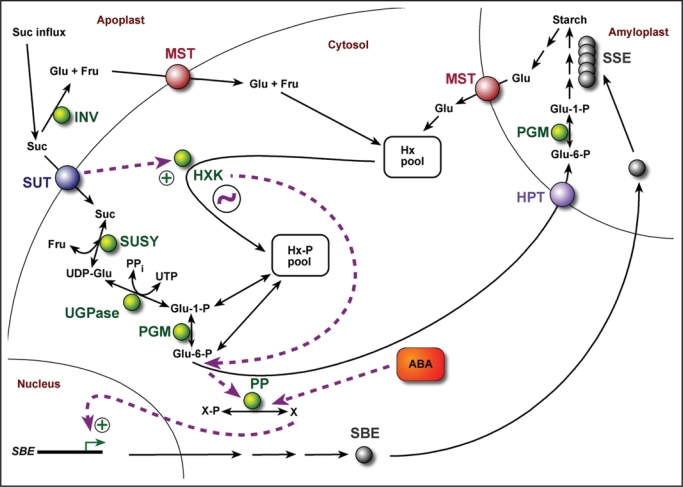
The findings in this work are summarized in a model of the sugar-induced signal transduction pathway that controls SBE expression in the cassava storage root sink (Fig. 6). Sucrose arriving in the sieve elements is translocated to the sink cells via a SUT. Some of the sucrose might be converted to glucose and fructose by apoplastic invertase, with subsequent uptake of the hexoses through monosaccharide transporters (MSTs). The SUT-substrate complex transmits a signal to HXK, activating the enzyme above a basal constitutive level. A similar signal might originate at the engaged MST. In both cases, the rationale is to signal to the cell that carbon has been allocated from the source to the sink for starch synthesis, and that glucose-6-P is in demand for transport to the amyloplast. From glucose-6-P, or a downstream metabolite, the signal eventually leads to activation of a protein phosphatase. The ensuing events are likely to involve migration of a transcription factor to the nucleus, followed by activation of the SBE genes.
Figure 6.
Model showing sugar and ABA signaling transduction (dashed purple arrows) during regulation of SBE gene expression in the cassava storage root. Sugar signaling is predominantly activated by the entry of sucrose. A signal is transmitted from the SUT to glucose-6-P, or a downstream regulator, via HXK. The sugar and ABA signaling pathways intersect at a protein phosphatase. The semidian oscillator (circled squiggle) is functionally associated with HXK. ABA, abscisic acid; Fru, fructose; Glu, glucose; HPT, hexose phosphate transporter; HXK, hexokinase; Hx, hexose; Hx-P, hexose-phosphate; INV, apoplastic invertase; MST, monosaccharide transporter; PGM, phophoglucomutase; PP, protein phosphatase; PPi, pyrophosphate; SBE, starch branching enzyme; SSE, starch synthesizing enzymes; SUSY, sucrose synthase; SUT, sucrose transporter; UGPase, UDP-glucose pyrophosphorylase; X, unknown protein.
Whereas the sugar-signaling pathway serves to regulate SBE expression in response to photosynthate status, ABA signaling would communicate stress conditions. It is known that environmental factors such as temperature and length of water deficit affect root expansion in cassava, and it has been proposed that ABA plays a critical role in the regulatory network that is activated by drought.9,34 Under such circumstances, or when the sink cell experiences osmotic stress due to high influx of sugars, conditions are not favorable for continued photosynthesis and vegetative growth and the plant would downregulate source activities and upregulate sink activities. From their work on regulation of starch synthesis in Arabidopsis leaves,31 suggested a model where ABA signaling increases the sensitivity of metabolic processes to a separate sugar signal. High sugar levels and other high osmotic conditions result in ABA accumulation and induces a “storage mode”, while in the absence of ABA the plant switches to a “mobilization mode”. In our model, the cross talk between sugar and ABA signaling merges at the protein phosphatase. Since induction of SBE expression by sucrose and ABA in combination was higher than for sucrose alone, it is quite possible that ABA boosts the sugar signal by interacting with the protein phosphatase. The placement of ABA activity downstream of HXK follows from the observation that signaling by both ABA and glucose-1-P circumvented the oscillator, and from the fact that ABA induction was insensitive to HXK inhibition.
The nature of the semidian oscillator is unknown but we hypothesize that it is functionally linked to HXK; semidian rhythmicity of SBE expression was obtained after induction with sucrose, turanose and glucose but not glucose-1-P or ABA. Conceivably, an oscillation in HXK activity could be dictated by a transcriptional rhythm for a HXK-interacting protein in very much the same way as described by Jones and Ort35 for the circadian regulation of sucrose phosphate synthase (SPS) activity in tomato leaves. The authors showed that the activity of SPS was dependent on reversible phosphorylation and subject to both diurnal and circadian control, and they inferred that an endogenous rhythm in the transcription of a protein phosphatase promoted the circadian oscillation of the SPS phosphorylation state. Interestingly, a 12-h endogenous rhythm for SPS activity was reported for soybean leaves.35,36 The diurnal behavior of SBE transcription and SBE activity in the storage roots of intact cassava plants and the existence of a local semidian oscillator in the storage root cells suggest that the temporal regulation of SBE expression comprises multiple levels of modulation.
Arguably, our studies on isolated storage root discs suffer from several shortcomings with regards to transposing the results to the whole plant level. For example, we do not know if phloem unloading in cassava is apoplastic or symplastic and, hence, to what extent the apoplastic uptake of sucrose that occurs in the root discs represents the normal transfer of solutes in cassava. In potato, phloem unloading shifts from essentially apoplastic during stolon elongation to predominantly symplastic during tuberization.37,38 Obviously, it will be important to determine the principles for phloem unloading in the cassava plant.
Another issue concerns the physiological significance of the semidian (and diurnal) fluctuations of SBE gene expression in the cassava storage root. It has been shown recently that SBE activity in wheat amyloplasts and chloroplasts is regulated by phosphorylation.39 If this is the same in cassava, and how such an allosteric regulation would cooperate with the semidian oscillation of SBE expression during development of the cassva storage root, remains to be investigated.
There are also questions with regards to the metabolic fluxes proposed in Figure 6 that need to be addressed in future experiments. Although it is well established that UGPase is involved in production of Glu-1-P,40–42 in their work on transgenic potatoes Zrenner et al.,43 suggested that only a minor portion of UGPase activity is required for normal carbon metabolism and starch synthesis in tubers. Thus to what extent UGPase contributes to Hx-P pool levels in the cassava storage root is not yet clear. Further, we are assuming in our model that the predominant form of carbon uptake in cassava amyloplasts is Glu-6-P as is the case in potato tubers, and not ADP-Glu as in cereals seeds (see recent review by Geigenberger and Fernie44). That, of course, needs to be firmly established.
In conclusion, we have uncovered a novel feature in the regulation of starch synthesis, an endogenous semidian sink oscillator, and present a model of the sugar-signaling network that controls SBE expression in the developing cassava storage root. We hope the model provides a framework for further studies in plant sugar signaling and sink-source communication.
Materials and Methods
Plant material and growth conditions.
Manihot esculenta Crantz cv. MH95/0414, officially designated as NASE 12 in Uganda, was grown in a glasshouse under a 14-h light, 23°C/10-h dark, 18°C (LD) regimen. Unless stated otherwise, growth conditions were as described.11 Cassava storage roots were sampled at 360 d.a.p. for transcript, protein and carbohydrate analyses.
RNA gel blot analysis.
Total RNA extraction from cassava storage roots and RNA gel blot analysis was carried out as described by Baguma et al.11
Zymogram analysis.
Zymograms for SBE activity were performed as described by Sun et al.12
Sugar and starch analyses.
Sugars were extracted by the ethanol method.13 Estimation of sugar and starch content was done by measurement of changes in NADPH concentration at A340 using the Enzytec™Kits (Scil Diagnostics GmbH, Sweden).
Induction of SBE expression.
360-day-old plants were entrained to dark conditions for 48 h. In series, cassava storage roots were harvested, sliced into 5 mm discs and depleted of endogenous sugars in 3% mannitol (v/v) for 24 h. The discs were subsequently transferred to a stack of Whatman papers saturated with 200 mM sugars or sugar analogs, and/or 5 µM ABA. Glucosamine (0–100 mM) or okadaic acid (1 µM) were added as indicated.
Acknowledgements
This study was supported by grants from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), Sida/SAREC under the auspices of the BIO-EARN program, and in part by U. S. Department of Energy Contract DEAC02- 05CH11231 with Lawrence Berkeley National Laboratory.
Abbreviations
- ABA
abscisic acid
- HXK
hexokinase
- SBE
starch branching enzyme
- SUT
sucrose translocator
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5715
References
- 1.Jansson C. Sugar signaling mutants in Arabidopsis. In: Esser K, et al., editors. Progress in Botany. Berlin: Springer Verlag; 2004. pp. 42–52. [Google Scholar]
- 2.Rolland F, Baena Gonzalez E, Sheen J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annual Review of Plant Biology. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 3.Sheen J, Zhou L, Jang JC. Sugars as signaling molecules. Curr Opinions Plant Biol. 1999;2:410–418. doi: 10.1016/s1369-5266(99)00014-x. [DOI] [PubMed] [Google Scholar]
- 4.Smeekens S. Sugar-Induced Signal Transduction in Plants. Ann Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Sun C, Olsson H, Mangelsen E, Höglund AS, Jansson C. Antisense ODN inhibition as a potent strategy in plant biology: identification of SUSIBA2 as a transcriptional activator in plant sugar signaling. Plant J. 2005;44:128–138. doi: 10.1111/j.1365-313X.2005.02515.x. [DOI] [PubMed] [Google Scholar]
- 6.Myers AM, Morell MK, James MG, Ball SG. Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol. 2000;122:989–997. doi: 10.1104/pp.122.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y. Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol. 2002;43:718–725. doi: 10.1093/pcp/pcf091. [DOI] [PubMed] [Google Scholar]
- 8.Smith AM. The biosynthesis of starch granules. Biomacromol. 2001;2:335–341. doi: 10.1021/bm000133c. [DOI] [PubMed] [Google Scholar]
- 9.Augusto A, Alves C. Cassava botany and physiology. In: Hillocks RJ, et al., editors. Cassava: Biology, Production and Utilization. London: CABI Publishing; 2002. pp. 67–89. [Google Scholar]
- 10.El-Sharkawy MA. Cassava biology and physiology. Plant Mol Biol. 2001;53:621–641. doi: 10.1007/s11103-005-2270-7. [DOI] [PubMed] [Google Scholar]
- 11.Baguma Y, Sun C, Ahlandsberg S, Mutisya J, Rubaihayo RP, Magambo MJ, Egwang TG, Larsson H, Jansson C. Expression patterns of the gene encoding starch branching enzyme II in the storage roots of cassava (Manihot esculenta Crantz) Plant Sci. 2003;164:833–839. [Google Scholar]
- 12.Sun C, Sathish P, Ek B, Jansson C. Demonstration of in vitro starch branching enzyme activity for a 51/52-kDa polypeptide isolated from developing barley (Hordeum vulgare) caryopses. Physiol Plant. 1996;96:474–483. [Google Scholar]
- 13.Veramendi J, Roessner U, Renz A, Willmitzer L, Trethewey RN. Antisense Repression of Hexokinase 1 Leads to an Overaccumulation of Starch in Leaves of Transgenic Potato Plants But Not to Significant Changes in Tuber Carbohydrate Metabolism. Plant Physiol. 1999;121:123–134. doi: 10.1104/pp.121.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolland F, Winderickx J, Thevelein J M. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem Sci. 2001;26:310–317. doi: 10.1016/s0968-0004(01)01805-9. [DOI] [PubMed] [Google Scholar]
- 15.Sun C, Palmqvist S, Olsson H, Borén M, Ahlandsberg S, Jansson C. A novel WRKY transcription factor, SUSIBA2, participates in sugar signaling in barley by binding to the sugar-responsive elements of the iso1 promoter. Plant Cell. 2003;15:2076–2092. doi: 10.1105/tpc.014597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C, Ridderstråle K, Larsson LG, Jansson C. Sweet delivery—Sugar translocators as ports of entry for antisense ODNs in plant cells. Plant J. 2007 doi: 10.1111/j.1365-313X.2007.03287.x. in press. [DOI] [PubMed] [Google Scholar]
- 17.Fernie AR, Willmitzer L, Trethewey RN. Sucrose to starch: a transition in molecular plant physiology. Trends Plant Sci. 2002;7:35–41. doi: 10.1016/s1360-1385(01)02183-5. [DOI] [PubMed] [Google Scholar]
- 18.Loreti E, De Bellis L, Alpi A, Perata P. Why and how do plant cells sense sugars? Annals Bot. 2001;88:803–812. [Google Scholar]
- 19.Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK. Extracellular invertase: key metabolic enzyme and PR protein. J Experiment Bot. 2003;54:513–524. doi: 10.1093/jxb/erg050. [DOI] [PubMed] [Google Scholar]
- 20.Sinha AK, Hofman MG, Römer U, Köckenberger W, Elling L, Roitsch T. Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 2002;128:1480–1489. doi: 10.1104/pp.010771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loreti E, Alpi A, Perata P. Glucose and disaccharide-sensing mechanisms modulate the expression of alpha-amylase in barley embryos. Plant Physiol. 2000;123:939–948. doi: 10.1104/pp.123.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atanassova R, Leterrier M, Gaillard C, Agasse A, Sagot E, Coutos Thèvenot P, Delrot S. Sugar-regulated expression of a putative hexose transport gene in grape. Plant Physiol. 2003;131:326–334. doi: 10.1104/pp.009522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnewald U, Herbers K. In: Plant Carbohydrate Biochemistry. Bryant JA, et al., editors. Oxford: Bios Scientific Publishers; 1999. pp. 69–78. [Google Scholar]
- 24.Chandran D, Reinders A, Ward JM. Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. J Biol Chem. 2003;278:44320–44325. doi: 10.1074/jbc.M308490200. [DOI] [PubMed] [Google Scholar]
- 25.Voitsekhovskaya OV, Heber U, Wiese C, Lohaus G, Heldt HW, Gamalei YV. Energized Uptake of Sugars from the Apoplast of Leaves: A Study of Some Plants Possessing Different Minor Vein Anatomy. Russian J Plant Physiol. 2002;49:44–53. [Google Scholar]
- 26.Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang JC, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelstein RR, Gibson SI. ABA and sugar interactions regulating development: cross-talk or voices in a crowd? Curr Opinions Plant Biol. 2001;5:26–32. doi: 10.1016/s1369-5266(01)00225-4. [DOI] [PubMed] [Google Scholar]
- 29.Gibson SI. Sugar and phytohormone response pathways: navigating a signalling network. J Experiment Bot. 2004;55:253–264. doi: 10.1093/jxb/erh048. [DOI] [PubMed] [Google Scholar]
- 30.León P, Sheen J. Sugar and hormone connections. Trends Plant Sci. 2003;8:110–116. doi: 10.1016/S1360-1385(03)00011-6. [DOI] [PubMed] [Google Scholar]
- 31.Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J. 2001;26:421–433. doi: 10.1046/j.1365-313x.2001.2641043.x. [DOI] [PubMed] [Google Scholar]
- 32.Busk PK, Pagès M. Regulation of abscisic acid-induced transcription. Plant Mol Biol. 1998;37:425–435. doi: 10.1023/a:1006058700720. [DOI] [PubMed] [Google Scholar]
- 33.Finkelstein RR, Rock CD. In: The Arabidopsis Book. Somerville CR, Meyerowitz EM, editors. Rockville, MD: American Society of Plant Biologists; 2002. (2002) http:/./www.aspb.org/publications/arabidopsis/ [DOI] [Google Scholar]
- 34.Alves AC, Setter TL. Response of Cassava to Water Deficit: Leaf Area Growth and Abscisic Acid. Crop Sci. 2000;40:131–137. [Google Scholar]
- 35.Jones TL, Ort DR. Circadian Regulation of Sucrose Phosphate Synthase Activity in Tomato by Protein Phosphatase Activity. Plant Physiol. 1997;113:1167–1175. doi: 10.1104/pp.113.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr PS, Rufty TW, Jr, Huber SC. Endogenous rhythms in photosynthesis, sucrose phosphate synthase activity and stomatal resistance in leaves of soybean (Glycine max [L] Merr.) Plant Physiol. 1985;77:275–280. doi: 10.1104/pp.77.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernie AR, Roessner U, Geigenberger P. The contribution of plastidial phosphoglucomutase to the control of starch synthesis within the potato tuber. Plant Physiol. 2001;125:1967–1977. doi: 10.1104/pp.125.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viola R, Roberts AG, Haupt S, Gazzani S, Hancock RD, Marmiroli N, Machray GC, Oparka KJ. Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading. Plant Cell. 2001;13:385–398. doi: 10.1105/tpc.13.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tetlow IJ, Wait R, Zhenxiao L, Akkasaeng R, Bowsher CG, Esposito S, Kosar-Hashemi B, Morell MK, Emes MJ. Protein Phosphorylation in Amyloplasts Regulates Starch Branching Enzyme Activity and Protein-Protein Interactions. Plant Cell. 2004;406:2131–2145. doi: 10.1105/tpc.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geigenberger P, Stitt M, Fernie AR. Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers. Plant Cell Environ. 2004;27:655–673. [Google Scholar]
- 41.Lytovchenko A, Hajirezaei M, Eikmeier I, Mittendorf V, Sonnewald U, Willmitzer L, Fernie AR. Expression of an Escherichia coli phosphoglucomutase in potato (Solanum tubersosum L.) results in minor changes in tuber metabolism and a considerable delay in tuber sprouting. Planta. 2005;221:915–927. doi: 10.1007/s00425-005-1490-z. [DOI] [PubMed] [Google Scholar]
- 42.Kleczkowski LA, Geisler M, Ciereszko I, Johansson H. UDP-glucose pyrophosphorylase. An old protein with new tricks. Plant Physiol. 2004;134:912–918. doi: 10.1104/pp.103.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zrenner R, Willmitzer L, Sonnewald U. Analysis of the expression of potato uridinediphosphate- glucose pyrophosphorylase and its inhibition by antisense RNA. Planta. 1993;190:247–252. doi: 10.1007/BF00196618. [DOI] [PubMed] [Google Scholar]
- 44.Geigenberger P, Fernie AR. Starch synthesis in the potato tuber. In: Hui YH, editor. Food Biochemistry and Food Processing. Oxford: Blackwell Publishing; 2006. pp. 253–270. [Google Scholar]