Abstract
After germination, seedlings follow one of two developmental programs, photo- and skotomorphogenesis, and the choice is determined by the interplay between environmental signals (light) and endogenous cues (the plant hormones gibberellins among others). In the December issue of Plant Journal we describe a molecular mechanism that allows the integration of light and gibberellin signaling to tightly control the switch between skoto- and photomorphogenesis. On one hand, the stability of HY5, a transcription factor required by light to promote photomorphogenesis, is enhanced in the light and in situations with compromised GA biosynthesis. And, on the other hand, the promotion of growth during etiolation is exerted by the PIF family of transcription factors, whose abundance is enhanced by the absence of light, and whose activity is regulated by functional interaction with gibberellin signaling. In this addendum we propose that the control of the activity of light-dependent transcription factors by gibberellins is a common theme in other developmental processes, such as shade avoidance and photoperiodic regulation of cell expansion.
Key words: gibberellins, photomorphogenesis, shade avoidance, cell expansion
Photomorphogenesis is the developmental program that very young seedlings follow if germination occurs in full light, and is characterized by reduced growth of the hypocotyl, expansion of the cotyledons, and the upregulation of the large set of genes required for the generation of new organs, photosynthesis and photoprotection, among other processes. On the other hand, skotomorphogenesis results in etiolated growth, with long hypocotyls and the formation of an apical hook with folded cotyledons.1
The switch between these two alternative developmental programs is largely regulated by light.2 In darkness, the activity of the E3 ubiquitin ligase encoded by COP1 maintains low levels of a series of transcriptions factors (HY5, HYH, HFR1, LAF1 and others)2–5 required for the promotion of light-induced traits. Furthermore, etiolated growth depends on the activity of the PIF transcription factors, which are relatively abundant in darkness, and are rapidly degraded upon illumination.6 This developmental transition is also modulated by at least two plant hormones, brassinosteroids (BR) and gibberellins (GA).7,8 The involvement of hormones is based on the observation that seedlings deficient in BR or GA synthesis or signaling are de-etiolated in darkness (i.e., they display short hypocotyls, unfolded cotyledons and derepression of light-regulated genes).7–9
To unveil the molecular mechanism that would account for the interaction between light and GA in the control of photomorphogenesis, we hypothesized that different light-regulated transcription factors (HY5, PIF3, etc.) would also be targets for GA signaling, thus acting as nodes for the integration of environmental and endogenous cues.10 To test this hypothesis, we examined the sensitivity of loss- and gain-of-function mutants of these transcription factors with respect to the de-etiolation induced by lack of GA. In fact, we found that hy5 mutants were more resistant to the de-etiolation in darkness caused by blocking GA biosynthesis, as indicated by their deficient unfolding of cotyledons, hypocotyl growth arrest and derepression of CAB2, compared to wild-type seedlings. Consistently, the opposite phenotype was found in HY5 gain-of-function mutants. The conclusion that GA regulate photomorphogenesis in part through HY5 was further supported by the observation that HY5 protein accumulated in GA deficient conditions in darkness, the same as had been reported for cop1 mutants.
On the contrary, loss-of-function mutations in PIF3 and in other PIF transcription factors caused increased sensitivity to the lack of GA, because these mutants displayed traits of enhanced de-etiolation in darkness compared to the wild type, when GA biosynthesis was compromised. This effect was consistent with a role of PIF proteins in promoting etiolated growth, and suggested that GA would promote skotomorphogenesis by enhancing the stability and/or activity of the PIF transcription factors. Although the stability of PIF3 was not affected by GA, we found that the expression of several target genes of PIF3 was severely affected in GA deficient conditions, indicating that, unlike HY5, GA do not regulate PIF stability, but PIF activity. Indeed, the most likely mechanism for this regulation is the physical interaction between DELLA proteins—which are GA-signaling elements that accumulate in the absence of GA—and the PIF transcription factors, as reported at least for light-grown seedlings.11
In summary, we propose that GA promote etiolated growth after germination in darkness by at least two mechanisms: on the one side, GA repress photomorphogenesis by maintaining low levels of HY5, and on the other side they keep low levels of DELLA proteins, which in turn are negative regulators of the PIF transcription factors that promote diverse aspects of etiolated growth.
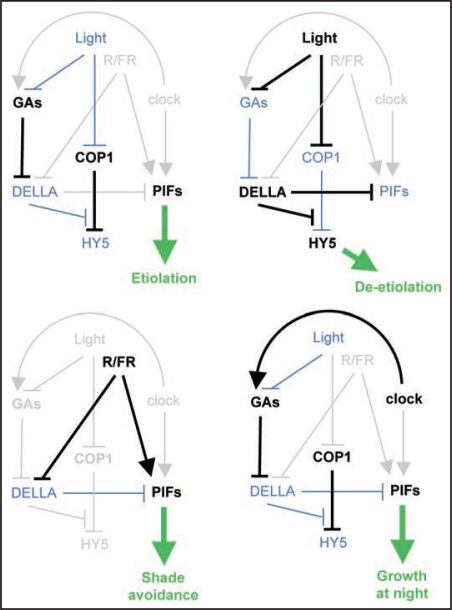
How specific is this interplay of light and GA for the switch between skoto- and photomorphogenesis? Light and GA participate in the regulation of development in many other stages of the plant's life cycle (Fig. 1). For instance, the ratio between red and far-red light is measured by the plants to determine the presence of neighbors and to promote shade avoidance to maximize light absorption.12 This response involves enhanced elongation of hypocotyls, petioles and stems to ”move“ the plant away from the shading effect of neighbors. Remarkably, both GA13 and the PIF proteins14 have been implicated in this process, and it wouldn't be surprising that part of the mechanism that enhances growth is caused by the release of the inhibitory interaction between the DELLA and PIF proteins. Another example may occur on a daily basis, in the regulation of the rhythmic hypocotyl growth. The period of maximum cell expansion in the hypocotyl in Arabidopsis is phased towards the end of the night. This is accomplished by a cooperative, negative effect of light signaling and the circadian clock on the PIF4 and PIF5 protein and transcript levels, respectively, which gates the activity of these growth promoting transcription factors towards the end of the dark period.15 Since GA also promote hypocotyl growth, it is tempting to speculate that the GA pathway is also under clock control in order to add robustness to the rhythmic growth response. This hypothesis is supported by the observation that GA biosynthesis is higher in night than in daylight periods in sorghum.16
Figure 1.
Molecular interactions between light and gibberellins during plant development. Arrows and bars indicate positive and negative effects respectively. In gray are shown the interactions that are not relevant for each developmental process. Thicker lines are used to depict the occurring activations or inhibitions.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5556
References
- 1.Neff MM, Fankhauser C, Chory J. Light: an indicator of time and place. Genes Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- 2.Yang J, Lin R, Sullivan J, Hoecker U, Liu B, Xu L, Deng XW, Wang H. Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell. 2005;17:804–821. doi: 10.1105/tpc.104.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duek PD, Elmer MV, van Oosten VR, Fankhauser C. The degradation of HFR1, a putative bHLH class transcription factor involved in light signaling, is regulated by phosphorylation and requires COP1. Curr Biol. 2004;14:2296–2301. doi: 10.1016/j.cub.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Seo HS, Yang JY, Ishikawa M, Bolle C, Ballesteros ML, Chua NH. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature. 2003;423:995–999. doi: 10.1038/nature01696. [DOI] [PubMed] [Google Scholar]
- 5.Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002;16:1247–1259. doi: 10.1101/gad.969702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorrain S, Genoud T, Fankhauser C. Let there be light in the nucleus! Curr Opin Plant Biol. 2006;9:509–514. doi: 10.1016/j.pbi.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- 8.Alabadi D, Gil J, Blazquez MA, Garcia-Martinez JL. Gibberellins repress photomorphogenesis in darkness. Plant Physiol. 2004;134:1050–1057. doi: 10.1104/pp.103.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, Harberd NP. DELLAs Contribute to Plant Photomorphogenesis. Plant physiology. 2007;143:1163–1172. doi: 10.1104/pp.106.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alabadi D, Gallego-Bartolome J, Garcia-Carcel L, Orlando L, Rubio V, Martínez C, Frigerio M, Iglesias-Pedraz JM, Espinosa A, Deng XW, Blázquez MA. Gibberellins modulate light signaling pathways to prevent Arabidopsis seedling de-etiolation in darkness. Plant J. 2008;53:324–335. doi: 10.1111/j.1365-313X.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 11.de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008 doi: 10.1038/nature06520. In press. [DOI] [PubMed] [Google Scholar]
- 12.Franklin KA, Whitelam GC. Phytochromes and shade-avoidance responses in plants. Ann Bot. 2005;96:169–175. doi: 10.1093/aob/mci165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djakovic Petrovic T, de Wit M, Voesenek LA, Pierik R. DELLA protein function in growth responses to canopy signals. Plant J. 2007;51:117–126. doi: 10.1111/j.1365-313X.2007.03122.x. [DOI] [PubMed] [Google Scholar]
- 14.Lorrain S, Allen T, Duek P, Whitelam GC, Fankhauser C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 15.Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- 16.Lee IJ, Foster KR, Morgan PW. Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 1998;116:1003–1011. doi: 10.1104/pp.116.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]