Abstract
Hydrotropism, the differential growth of plant roots directed by a moisture gradient, is a long recognized, but not well-understood plant behavior. Hydrotropism has been characterized in the model plant Arabidopsis. Previously, it was postulated that roots subjected to water stress are capable of undergo water-directed tropic growth independent of the gravity vector because of the loss of the starch granules in root cap columella cells and hence the loss of the early steps in gravitropic signaling. We have recently proposed that starch degradation in these cells during hydrostimulation sustain osmotic stress and root growth for carrying out hydrotropism instead of reducing gravity responsiveness. In addition, we also proposed that abscisic acid (ABA) and water deficit are critical regulators of root gravitropism and hydrotropism, and thus mediate the interacting mechanism between these two tropisms. Our conclusions are based upon experiments performed with the no hydrotropic response (nhr1) mutant of Arabidopsis, which lacks a hydrotropic response and shows a stronger gravitropic response than that of wild type (WT) in a medium with an osmotic gradient.
Key words: starch, water deficit, auxin, abscisic acid, gravitropism, hydrotropism
Roots of land plants sense and respond to different stimuli, some of which are fixed in direction and intensity (i.e., gravity) while other vary in time, space, direction and intensity (i.e., obstacles and moisture gradients). Directed growth of roots in relation to a gradient in moisture is called hydrotropism and begins in the root cap with the sensing of the moisture gradient. However, since gravity is an omnipresent accompaniment of Earthly life and many living process have evolved with it as a background constant, it is not surprising that root hydrotropism interacts with gravitropism.1 The hydrotropic response in Arabidopsis, compare with other plants such as pea and cucumber2,3 is readily observed even in the presence of gravity.4,5 When Arabidopsis roots are subjected to a water gradient, such that the source of water is placed 180° opposed to the gravity vector, the roots will grow upwards, displaying positive hydrotropism. Therefore, it has been feasible to isolate so far two Arabidopsis mutants affected in their hydrotropic response.5,6 Analysis of these mutants reveals new insights of the mechanism of hydrotropism. For one hand, the no hydrotropic response (nhr1) mutant lacks a hydrotropic response, and shows a stronger gravitropic response than that of wt and a modified wavy growth response in a medium with an osmotic gradient.5,7 On the other hand, the mizu-kussei1 (miz1) mutant did not exhibit hydrotropism and showed regular gravitropism.6 Hence, the root hydrotropic response is both linked and unlinked from the gravitropic one. Nonetheless, miz1 roots also showed a reduced phototropism and a modified wavy growth response. This indicates that both MIZ1 and NHR1 are not exclusive components of the mechanism for hydrotropism and supports the notion that the root cap has assessment mechanisms that integrate many different environmental influences to produce a final integrated response.8 Thus, the physiological phenomena distinctively displayed by roots in order to forage resources from the environment are the result of integrated responses that resulted from many environmental influences sensed in the root cap.
In the course of studying how gravity and water availability affected the perception and assessment of each other in root cap cells that generated the final root tropic response, we found that ABA is a critical regulator of the signal transduction mechanism that integrated these two-root tropisms.7 For this, we analyzed the long-term hydrotropic response of Arabidopsis roots in an osmotic gradient system. ABA, locally applied to seeds or root tips of nhr1, significantly increased root downward growth in a medium with an osmotic gradient (root length of nhr1 seedlings grown in this medium were on average 12.5 mm and plus 10 µM ABA were 25.1 mm). On the other hand, WT roots germinated and treated locally with ABA in this system were strongly gravitropic, albeit they had almost no starch in amyloplasts of root cap columella cells. Hydrotropically stimulated nhr1 roots, with or without ABA, maintained starch in amyloplastas, as opposed to those of WT. Therefore, the near-absence (WT) or abundant presence (nhr1) of starch granules does not affect the extent of downward gravitropism of roots in an osmotic gradient medium. Starch degradation in the wt might participate in osmoregulation by which root cells maintain turgor and consequently carry out hydrotropism, instead of reducing gravity responsiveness. In fact, it was just recently published that salt-induced rapid degradation of starch in amyloplasts is not likely the main reason for a negative gravitropic response seen under salt stress, because sos mutant roots of Arabidopsis showed negative gravitropic growth without any apparent rapid digestion of starch granules.9 Additionally, the stems of overwintering tubers of Potamogeton pectinatus are capable of elongating much faster in the absence than in the presence of oxygen for up to 14 days and its stems has an enhanced capacity for gravitropic movements in completely anoxic conditions.10 These authors hypothesized that ABA and starch degradation in the starchy tuber sustained stem cell elongation and cell division as well as differential growth required for the gravitropic response in these aquatic plants. These data taken together suggest that in conditions of anoxia, or water stress, ABA and degradation of starch play a critical role in the ability to survive relatively prolonged periods of unfavorable growth conditions. These players are critical when water or minerals are scarce since they regulate the enhancement of root downward growth. However, since roots can trail humidity gradients in soil, they can modulate their branching patterns (architecture) and thus respond to hydrotropism once a water-rich patch is found. Then the response of plants to gravity is principally one of nutrition (shoots to light, roots to mineral and water) and consequently must be regulated according to the long- and short-term environmental variables that occur during the development of the plant.
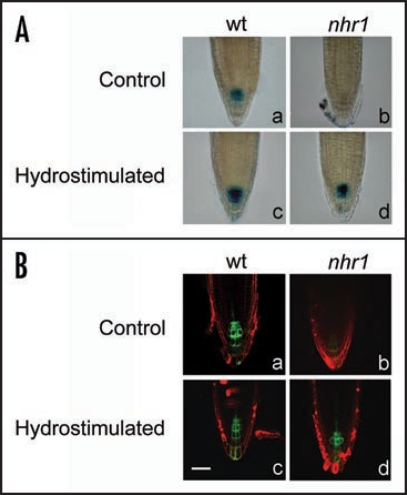
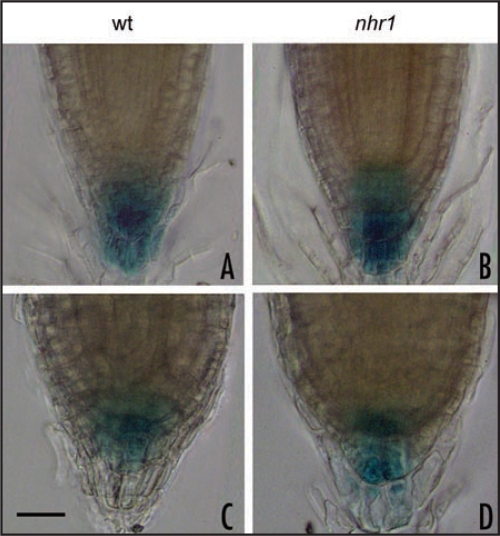
Differential growth that occurs during the gravitropic and phototropic response has been explained according to the Cholodny-Went hypothesis, which states that the lateral transport of auxin across stimulated plant tissues is responsible for the curvature response.11 Analysis of hydrotropism in some Arabidopsis agravitropic auxin transport mutants has demonstrated that these mutations do not influence their hydrotropic response.4 Furthermore, current pharmacological studies using inhibitors also indicated that both auxin influx and efflux are not required for hydrotropic response whereas auxin response is necessary for it.12 These authors suggested a novel mechanism for auxin in root hydrotropism. Here, we analyzed whether asymmetric auxin distribution takes place across hydrotropically-stimulated roots using transgenic plants carrying a responsive auxin promoter (DR5) driving the expression of β-glucuronidase (GUS) or green fluorescent protein (GFP)13,14 in wt and nhr1 backgrounds. Wt and nhr1 roots hydrotropically stimulated in a system with air moisture gradient5 showed no asymmetric expression of the DR5:: GUS or DR5::GFP (Fig. 1A and B). Nonetheless, nhr1 roots showed a substantial decrease in the signal driven by the DR5::GUS and GFP reporters in humidity saturated conditions (Fig. 1A, part b and B, part b), which might indicate that auxin-induced gene expression in the root cap was inhibited. It remains to be determined the significance of this inhibition in the no hydrotropic response phenotype displayed by nhr1 roots. Determination of the DR5::GUS expression in wt and nhr1 roots growing in an osmotic gradient medium for testing long-term hydrotropism revealed that the GUS signal was to some extent diminished in both wt or in nhr1 roots (Fig. 2C and D) compared to those roots growing in normal medium (Fig. 2A and B). An inhibitor of auxin response reduced hydrotropism,12 and also inhibited auxin-dependent DR5::GUS expression.15 However, a decrease of DR5::GUS in wt root tips was not an impediment for developing an hydrotropic response. On the other hand, nhr1 roots also showed a decrease of DR5::GUS expression (Fig. 2B and D) and a complete absence of DR5::GFP (data not shown), which did not influence the extent of downward root gravitropism in water deficit conditions. Therefore, it is difficult to assign a role of auxin-induce gene expression in hydrotropism and further studies are required in order to unravel this issue. Furthermore, it needs to be resolved whether these expression studies oppose the idea that gradients in auxin precede differential growth in response to humidity gradients.
Figure 1.
DR5:: GUS (A) and DR5::GFP (B) activity in the wild type NHR1 and nhr1 backgrounds. (A) Root tips hydrostimulated in a system with air moisture gradient (C and D) or grown in a saturated water conditions (A and B) stained with 1 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronic (X-Gluc) acid buffer under the same conditions for 80 min. (B) Root tips hydrostimulated as in (A) (C and D) or grown in a saturated water conditions (A and B) whose green fluorescent signal was visualized by confocal microscopy. Shown are images selected from at least 45 representative root tips. Bar = 29 µm.
Figure 2.
Expression of DR5::GUS in wild type NHR1 and nhr1 backgrounds. Roots were hydrotropically stimulated for 8 days in a medium with an osmotic gradient (C and D) or grown in normal medium (A and B) and stained with X-Gluc acid buffer under the same conditions for 80 min. Shown are images selected from at least 50 representative root tips. Bar = 25 µm.
Our studies7 revealed that ABA is a critical regulator of both root gravitropism and hydrotropism in water deficit conditions, and that the role of auxin under these conditions seems to differ from those observed in several studies thus far published on gravitropism made under well-water conditions. The molecular characterization of NHR1 and from other nhr-like mutants already isolated in our lab will clarify the mechanisms involved in this fascinating tropism.16
Acknowledgements
This work was supported by the Consejo Nacional de Ciencias y Tecnología (Grant No. 46022Q) and the University of California Institute for Mexico and United States.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5672
References
- 1.Cassab GI. Other tropisms and their relationship to gravitropism. In: Gilroy S, Masson PH, editors. Plant Tropisms. Iowa, IA, USA: Blackwell Publishing; 2008. pp. 123–139. [Google Scholar]
- 2.Takahashi H, Suge H. Root hydrotropism of an agravitropic mutant, ageotropum. Physiol Plant. 1991;82:24–31. [Google Scholar]
- 3.Mizuno H, Kobayahi A, Fujii N, Yamashita M, Suge H. Induction of hydrotropism in clinorotated pea seedling roots of Alaska pea, Pisum sativum L. J Plant Res. 1996;109:335–337. doi: 10.1007/BF02344481. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N, Goto N, Okada K, Takahashi H. Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta. 2002;43:793–801. doi: 10.1007/s00425-002-0840-3. [DOI] [PubMed] [Google Scholar]
- 5.Eapen D, Barroso ML, Campos ME, Ponce G, Corkidi G, Dubrovsky JG, Cassab GI. A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis. Plant Physiol. 2003;131:536–546. doi: 10.1104/pp.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi A, Takahashi A, Kakimoto Y, Miyasawa Y, Fujii N, Higashitani A, Takahashi H. A gene essential for hydrotropism in roots. Proc Natl Acad Sci USA. 2007;104:4724–4729. doi: 10.1073/pnas.0609929104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponce G, Rasgado FA, Cassab GI. Roles of amyloplasts and water deficit in root tropisms. Plant Cell Environ. 2008;31:205–217. doi: 10.1111/j.1365-3040.2007.01752.x. [DOI] [PubMed] [Google Scholar]
- 8.Trewavas A. Aspects of plant intelligence. Ann Bot. 2003;92:1–20. doi: 10.1093/aob/mcg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun F, Zhang W, Hu H, Li B, Wang Y, Zhao Y, Li K, Liu M, Li X. Salt modulates gravity signaling pathway to regulate growth direction of primary roots in Arabidopsis. Plant Physiol. 2008;146:178–188. doi: 10.1104/pp.107.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Summers JE, Jackson MB. Anaerobic conditions strongly promote extension by stems of overwintering tubers of Potamogeton pectinatus L. J Exp Bot. 1994;45:1309–1318. [Google Scholar]
- 11.Muday GK, Rahman A. Auxin transport and the integration of gravitropic growth. In: Gilroy S, Masson PH, Iowa IA, editors. Plant Tropisms. USA: Blackwell Publishing; 2008. pp. 47–77. [Google Scholar]
- 12.Kaneyasu Y, Kobayashi A, Nakayama M, Fujii N, Takahashi H, Miyazawa Y. Auxin response, but not its polar transport, plays a role in hydrotropism of Arabidopsis roots. J Exp Bot. 2007;58:1143–1150. doi: 10.1093/jxb/erl274. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Yang WA, Guilfoyle TJ. An Auxin-responsive promoter is differentially induced by auxin gradients during tropisms. Plant Cell. 1991;3:1167–1175. doi: 10.1105/tpc.3.11.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottenschläger IP, Wolff P, Wolverton RP, Bhalerao P, Sandberg G, Ishikawa H, Evans M, Palme K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA. 2003;100:2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oono Y, Oura C, Rahman A, Aspuria ET, Hayashi K, Tanaka A, Uchimiya H. p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiol. 2003;133:1135–1147. doi: 10.1104/pp.103.027847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eapen D, Barroso ML, Campos ME, Ponce G, Cassab GI. Hydrotropism: root growth responses to water. Trends Plant Sci. 2005;10:44–50. doi: 10.1016/j.tplants.2004.11.004. [DOI] [PubMed] [Google Scholar]