Abstract
The fields of plant water relations and plant biomechanics have traditionally been studied separately even though often the same tissues are responsible for water transport and mechanical support. There is now increasing evidence that hydraulic and mechanical adaptations may influence one another. We studied the changes in the hydraulic and mechanical properties of the wood along lateral roots of two species of buttressed trees. In these roots, the mechanical contstraints quantified by strain measurements are known to decrease distally. Further, we investigated the effect of mechanical loading on the vessel anatomy in these and four other species of tropical trees. We found that as the strain decreased, the wood became progressively less stiff and strong but the conductivity increased exponentially. This was reflected in that adaptations towards re-enforcing mechanically loaded areas resulted in xylem with fewer and smaller vessels. In addition a controlled growth experiment on three tree species showed that drought adaptation may results in plants with stronger and stiffer tissue. Our results indicate that hydraulic and mechanical stress adaptations may be interrelated, and so support recent studied suggesting that physiological responses are complex balances rather than pure optimisations.
Key words: conductivity, modulus of elasticity, strain, tree ecophysiology, tropical trees, wood anatomy, yield stress
It is well known that the woody tissue of plants is responsible for carrying out several functions simultaneously, of which the two most important may be water transport and mechanical support. In spite of this, the fields of plant water relations and plant biomechanics have traditionally been studied separately (however, see refs. 1–4). An increasing number of studies now indicate that there may be interrelations between hydraulic and mechanical stress adaptations, both in the form of positive interactions and trade-offs. In the former case, adaptations with respect to one of the two stresses positively affect the plants ability to withstand the other. Drought adaptation, for instance, is associated with an increase in the density of the tissue, which may result in stiffer and stronger wood.5,6 Further, increasing the transectional area of the sapwood increases the conductivity as well as the rigidity of the plant and may occur as an adaptation to either drought- or mechanical stress.7,8 In such cases, because of the importance of hydraulic sufficiency as well as adequate mechanical support, biomass must be partitioned to allow for both functions simultaneously even if this results in a surplus allocation with respect to one. In the case of trade-offs, the hydraulic or mechanical adaptations are detrimental to the plants' capabilities with respect to the other. Within the woody tissue, for instance, larger and more numerous vessels increase the conductivity but may weaken the wood.1–3,9–11 Analogously, hydraulic optimisation dictates an increase in the transectional sapwood area up though the plant, so that the summed area of all branches at a given height would be greater than that of the trunk12 but mechanical optimisation a decrease.13 In the case of physiological parameters within which there are trade-offs, achieving adequate design whilst minimising biomass allocation becomes complex,4 and a number of possible but less optimal solutions exist.
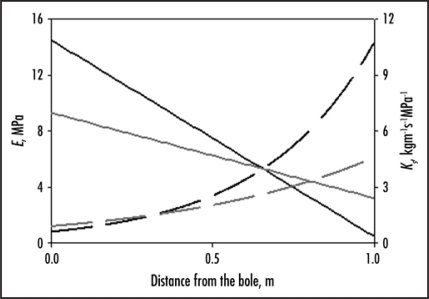
In our study,14 we looked at how mechanical as well as hydraulic parameters changed along the lateral roots of two tropical tree species, Tachigali melinonii and Xylopia nitida, both of which produce buttress roots. Along the roots of these species there is a strong distal decrease in the magnitude of the locally supported mechanical loads,15 making them ideal model organisms for investigating mechanical adaptation of the tissue and the impact this has on hydraulic parameters. We measured the density, conductivity, strength, stiffness, sapwood area and second moment of area at various points distally along the roots as well as in the lower trunk, and compared the values to those for strain. In both species, the strength and stiffness of the tissue decreased distally along the roots as the strain dropped, and the conductivity concurrently increased exponentially (Fig. 1). This appeared related to changes in the density of the wood; in both species, the density increased towards the bole and was positively correlated with mechanical properties but negatively with conductivity. As in previous studies, the distal most roots had a higher conductivity and lower stiffness and strength than that of the trunk. The proximal roots, however, had a lower conductivity but a greater strength and density. This indicates that the general pattern that roots have a higher conductivity than the stem cannot be explained by the water potential gradient from the soil to the leaves as often assumed, but instead by the differing mechanical requirements on these structures.
Figure 1.
The modulus of elasticity in bending, E, (solid lines) and the specific conductivity, Ks, (dashed lines) of the woody tissue of the lateral roots shown as a function of the distance from the bole. The trend-lines are shown for the buttressed tree species Tachigali melinonii (black) and Xylopia nitida (grey), and are based on data presented in Christensen-Dalsgaard, et al, 2007a.
These hydraulic and mechanical adaptations were well in accordance with anatomical adaptations seen for these as well as four other species from the same area representing two different rooting morphologies, for which the strain patterns differ. We found that the vessels were significantly smaller and less numerous in the xylem tissue of highly mechanically loaded compared to less loaded sections of the tree15 (Fig. 2). Further, the generally observed pattern16,17 that vessel size increases radially from the pit to the bark was not observed throughout the structure.18 In the proximal parts of the buttress roots, which are highly mechanically loaded throughout growth and development, the vessels maintained the small size found at the pith throughout the transect. In the distal parts of the buttress roots, in which the mechanical loading increases during growth, the vessels decreased rather than increased in size from the pith to the bark.18 Since the adaptation of the xylem tissue towards re-enforcing highly strained areas appear associated with changes in the vessel anatomy reducing the conductivity, radial changes in vessel size may not only be a function of cambial ageing, but also influenced by changes in stresses during growth.
Figure 2.
Tissue sections from the trunk and roots of the buttressed tree species Xylopia nitida, left, and the taproot anchored Oxandra asbeckii, right. All tissue sections are imaged at the same magnification; the width of each image is 4, 6 mm. In O. asbeckii, where the main rigid element resisting overturning is provided by the taproot and the lower bole, the trunk has smaller and fewer vessels than either of the root sections, as often described in litterature. In buttressed species such as X. nitida, on the other hand, where the area subjected to the greatest longitudinal strains and stresses is found in the proximal part of the buttress roots (marked in grey), this part of the root system is instead associated with fewer and smaller vessels than the trunk.
In addition to hydraulic effects of mechanical adaptations, the converse may occur; hydraulic adaptations could affect the mechanical properties of the tissue. Drought adapted plants have a greater resistance to cavitation,19,20 which appears related to an increased relative thickness of the conduit or the fibre cell walls,5,21 and so an increased wood density.5,22 However, since the mechanical properties are determined by the micro fibril angle (MFA) of the S2 cell wall layer as well as density,23 the data from the few studies measuring the mechanical effects of hydraulic adaptation have not found consistent effects.24,25 In these studies, complex natural systems were investigated making it difficult to distinguish between various climatic effects. We therefore grew seedlings from the three tree species Ochroma lagopus, Acacia karroo and Betula pendula under well-watered and droughted conditions, respectively, and measured the effect on the modulus of elasticity, the yield stress and the density (unpublished results). The stems of the droughted plants had a higher stiffness and strength than that of the well-watered plants. Only in O. lagopus, however, did the stems of the droughted seedlings have denser tissue than that of the well-watered seedlings; in B. pendula and A. karroo the mechanical differences were probably due to adaptations in the MFA instead. This was supported by that the density/elasticity ratio, which may be a good indicator of the MFA,23,26 was significantly higher in the well-watered than in the droughted plants.
Our work adds to the growing body of evidence indicating that there may be interrelations between hydraulic and mechanical stress adaptations.1–3,5,9–12,25 Modifications of the xylem towards mechanically reinforcing stressed or strained areas simultaneously impacted the conductive physiology of the trees studied, and drought adaptations resulted in stiffer and stronger tissue. This provides further evidence that in order to fully understand plant physiology and ecology it is necessary to consider the various functions of wood simultaneously and attempt to unravel causal relationships between e.g., the hydraulic and mechanical functioning of the tissue. Rather than being a matter of simple optimisations, adaptations towards one environmental stress will affect how plants adapt to the others. Because of the complexity of this balance, however, interrelations between parameters are not always found. For instance, mechanical strengthening due to hydraulic adaptations could reduce hydraulic effects of mechanical stresses; trees growing in climates with winter frost may be stronger due to larger amounts of sap- or heart wood and drought adaptation may result in a stiffer and stronger plant.21,24,27 This could explain why the relatively clear trade-off between hydraulic and mechanical parameters found in this and other studies have not always been found in non-tropical species.21,24,27–29 Deepening our knowledge on how multi-factor adaptation balances are affected by climatic conditions and the signalling processes involved in these complex processes would aid our understanding of ecophysiological responses in plants.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5673
References
- 1.Gartner BL. Is the climbing habit of poison oak ecotypic. Funct Ecol. 1991;5:696–704. [Google Scholar]
- 2.Gartner BL. Stem hydraulic-properties of vines vs shrubs of Western poison oak, Toxicodendron-Diversilobum. Oecologia. 1991;87:180–189. doi: 10.1007/BF00325255. [DOI] [PubMed] [Google Scholar]
- 3.Gartner BL. Structural stability and architecture of vines vs shrubs of poison oak, Toxicodendron-Diversilobum. Ecol. 1991;72:2005–2015. doi: 10.1007/BF00325255. [DOI] [PubMed] [Google Scholar]
- 4.Farnsworth KD, Niklas KJ. Theories of optimization, form and function in branching architecture in plants. Funct Ecol. 1995;9:355–363. [Google Scholar]
- 5.Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloch KA. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia. 2001;126:457–461. doi: 10.1007/s004420100628. [DOI] [PubMed] [Google Scholar]
- 6.Niklas KJ. Mechanical properties of black locust (Robinia pseudoacacia) wood: Correlations among elastic and rupture moduli, proportional limit, and tissue density and specific gravity. Ann Bot. 1997;79:479–485. [Google Scholar]
- 7.Mencuccini M, Grace J. Climate influences the leaf-area sapwood area ratio in Scots pine. Tree Phys. 1995;15:1–10. doi: 10.1093/treephys/15.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Telewski FW. Wind-induced physiological and developmental responses in trees. In: Coutts MP, Grace J, editors. Wind and Trees. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 9.Hathaway RL, Penny D. Root strength in some Populus and Salix clones. NZ J Bot. 1975;13:333–344. [Google Scholar]
- 10.Beery WH, Ifju G, McLain TE. Quantitative wood anatomy - relating anatomy to transverse tensile-strength. Wood Fibre Sci. 1983;15:395–407. [Google Scholar]
- 11.Isnard S, Speck T, Rowe NP. Mechanical architecture and development in Clematis: implications for canalised evolution of growth forms. New Phytol. 2003;158:543–559. doi: 10.1046/j.1469-8137.2003.00771.x. [DOI] [PubMed] [Google Scholar]
- 12.McCulloh KA, Sperry JS, Adler OFR. Murray's law and the hydraulic vs mechanical functioning of wood. Funct Ecol. 2004;18:931–938. [Google Scholar]
- 13.Mattheck C, Bethge K, Schafer J. Safety factors in trees. J Theor Biol. 1993;165:185–189. [Google Scholar]
- 14.Christensen-Dalsgaard KK, Ennos AR, Fournier M. Changes in hydraulic conductivity, mechanical properties and density reflecting the fall in strain along the lateral roots of two species of tropical trees. J Exp Bot. 2007;58:4095–4105. doi: 10.1093/jxb/erm268. [DOI] [PubMed] [Google Scholar]
- 15.Christensen-Dalsgaard KK, Fournier M, Ennos AR, Barfod AS. Changes in vessel anatomy in response to mechanical loading in six species of tropical trees. New Phytol. 2007;176:610–622. doi: 10.1111/j.1469-8137.2007.02227.x. [DOI] [PubMed] [Google Scholar]
- 16.Ismail J, Jusoh MZ, Sahri MH. Anatomical variation in planted Kelempayan (Neolamarckia-Cadamba, Rubiaceae) IAWA Journal. 1995;16:277–287. [Google Scholar]
- 17.Lei H, Milota MR, Gartner BL. Between- and within-tree variation in the anatomy and specific gravity of wood in Oregon white oak (Quercus garryana Dougl) Iawa J. 1996;17:445–461. [Google Scholar]
- 18.Christensen-Dalsgaard KK, Ennos AR, Fournier M. Are radial changes in vascular anatomy mechanically indiced or an ageing process? Evidence from observations on buttressed tree root systems. Trees. (in press) [Google Scholar]
- 19.Sperry JS, Hacke UG, Oren R, Comstock JP. Water deficits and hydraulic limits to leaf water supply. Plant Cell Env. 2002;25:251–263. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 20.Pratt RB, Jacobsen AL, Ewers FW, Davis SD. Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phytol. 2007;174:1–12. doi: 10.1111/j.1469-8137.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen AL, Ewers FW, Pratt RB, Paddock WA, Davis SD. Do xylem fibers affect vessel cavitation resistance? Plant Phys. 2005;139:546–556. doi: 10.1104/pp.104.058404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beadle CL, Banham PW, Worledge D, Russell SL, Hetherington SJ, Honeysett JL, White DA. Effect of irrigation on growth and fibre quality of Eucalyptus globulus and Eucalyptus nitens. Appita J. 2001;54:144–147. [Google Scholar]
- 23.Evans R, Ilic J. Rapid prediction of wood stiffness from microfibril, angle and density. Forest Prod J. 2001;51:53–57. [Google Scholar]
- 24.Watt MS, Moore JR, Facon JP, Downes GA, Clinton PW, Coker G, Davis MR, Simcock R, Parfitt RL, Dando J, Mason EG, Bown HE. Modelling the influence of stand structural, edaphic and climatic influences on juvenile Pinus radiata dynamic modulus of elasticity. Forest Ecol Man. 2006;229:136–144. [Google Scholar]
- 25.Pratt RB, Jacobsen AL, Ewers FW, Davis SD. Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phytol. 2007;174:1–12. doi: 10.1111/j.1469-8137.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang JL, Evans R. Prediction of MOE of eucalypt wood from microfibril angle and density. Holz Als Roh-Und Werkstoff. 2003;61:449–452. [Google Scholar]
- 27.Jacobsen AL, Agenbag L, Esler KJ, Pratt RB, Ewers FW, Davis SD. Xylem density, biomechanics and anatomical traits correlate with water stress in 17 evergreen shrub species of the Mediterranean-type climate region of South Africa. J Ecol. 2007;95:171–183. [Google Scholar]
- 28.Mencuccini M, Grace J, Fioravanti M. Biomechanical and hydraulic determinants of tree structure in Scots pine: Anatomical characteristics. Tree Phys. 1997;17:105–113. doi: 10.1093/treephys/17.2.105. [DOI] [PubMed] [Google Scholar]
- 29.Woodrum CL, Ewers FW, Telewski FW. Hydraulic, biomechanical, and anatomical interactions of xylem from five species of Acer (Aceraceae) Am J Bot. 2003;90:693–699. doi: 10.3732/ajb.90.5.693. [DOI] [PubMed] [Google Scholar]