Abstract
The mitochondrion has a central role during programmed cell death (PCD) in animals, acting as both a sensor of death signals, and as an initiator of the biochemical processes which lead to the controlled destruction of the cell. In contrast to our extensive knowledge of animal cell death, the part played by mitochondria in the death of plant cells has received relatively little attention. Using a combination of whole-organism and cell-based models, we recently demonstrated that changes in mitochondrial morphology are an early and crucial step in plant cell death. Here, we discuss these findings in the light of recent literature, and how they relate to our knowledge of plant cell death as a whole.
Key words: mitochondria, cell death, mitochondrial dynamics, morphology
Plant Cell Death and the Mitochondrion
One of the first visible indicators of animal cells undergoing apoptosis (a form of PCD defined by set morphological criteria; see below) is a rapid change in mitochondrial morphology, caused by an upregulation of the mitochondrial division machinery.1,2 This change in morphology appears to aid the release of mitochondrial proteins (e.g., cytochrome c) into the cytosol, which in turn switches on a range of cellular proteases that carefully degrade the cell and its components (reviewed in ref. 3). Importantly, inhibiting the change in mitochondrial morphology prevents cell death, demonstrating that this change is vital to the apoptotic process.
To investigate whether mitochondria play a similar role in plants, we subjected Arabidopsis protoplasts expressing mitochondrial-targeted GFP4 to chemical (reactive oxygen species; ROS) or physical (a mild heat-shock) stresses to induce cell death. Within minutes, mitochondria in induced cells had undergone a rapid change in morphology, exhibiting an abnormal, swollen appearance (termed the mitochondrial morphology transition) that preceded death of the cell by many hours.5 This morphology transition was blocked by treatment, prior to application of either stress, with the superoxide dismutase analogue TEMPOL, indicating that ROS were involved. Similarly, pre-incubation with either lanthanum chloride (which blocks calcium flux), or cyclosporin A (an inhibitor of the proposed mitochondrial permeability transition pore) blocked both the mitochondrial morphology transition and subsequent cell death.5 From these results, we concluded that the mitochondrial morphology transition is an early and necessary component of the cell death process in plants.
These findings, along with other recent reports, are beginning to highlight the ubiquity of mitochondria morphology alterations in plant cell death. Using similar methodologies, Gao and co-workers reported that a mitochondrial morphology transition and cell death are induced by exposure to excess ultraviolet light (UV-C); again these changes can be attenuated by pre-incubation of cells with either antioxidants (in this case ascorbic acid) or cyclosporin A.6 In an earlier study, treatment of Arabidopsis protoplasts with protoporphyrin IX (a death-inducing photosynthetic substrate7) led to changes in the shape and cellular position of mitochondria prior to death.8 The mitochondrial morphology transition has also been reported to occur prior to the final stages of senescence in Medicago truncatula cell suspension cultures,9 indicating that multiple plant PCD pathways are controlled by, or contain, a mitochondrial morphology component.
Plants Versus Animals—The Comfort of Paradigms
One clear difference between animal and plant cell death is that the change in mitochondrial morphology during plant cell death does not appear to involve increased mitochondrial division, as there is neither the decrease in size, nor increase in number, of individual organelles that would be expected if this were the case.5,10 While slavishly following animal paradigms in this regard may be straightforward, it would seem that the difference between the morphology of wild-type plant mitochondria (typically a large number of small, discrete organelles per cell11) and wild-type animal mitochondria (typically a relatively small number of relatively long, interconnected organelles per cell), together with the reduced (or absent) requirement for cytochrome c release as an initiator of downstream processes,7,12 makes the initial mitochondrial division step unnecessary in plants. Indeed, many aspects of research into the biochemical and morphological bases of plant cell death have become wrapped up in the patterns and terminology derived from animal studies, which we suggest is detrimental to the progression of this important field.
One repeat offence in echoing the animal paradigm is the use of the term “apoptosis” (or even “apoptosis-like”) when referring to PCD in plants. Apoptosis is a process of cell degradation and removal defined by strict morphological characteristics, including condensation of the nucleus and cytoplasm into membrane-bound “apoptotic bodies”, and is concluded by the removal of these fragments by phagocytosis, carried out by a neighbouring cell.13,14 Given that important steps in the process do not occur in plants, it seems highly illogical to use the term to describe a plant phenomenon. The use of another word, “necrotic” (which usually refers to death caused by a sudden, uncontrollable event), to describe a death process also appears illogical as it classically defines the end of an event (i.e., a dead cell), rather than how the end is reached.13,15 In both cases, the danger is that researchers become fixated on fitting death programmes in plants to established patterns from the animal world. At best, this may lead to sub-optimal depictions of what is occurring in plants; at worst, it may blind inexperienced researchers from making important discoveries that could advance the field, as they try to match what they see to what is already known.
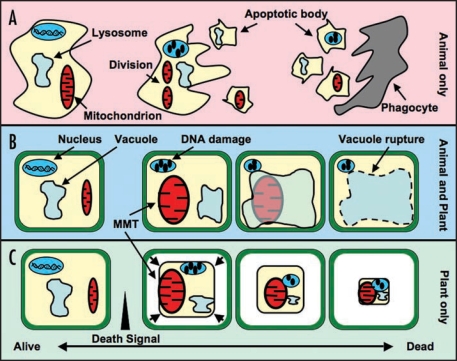
While the complexity of death programmes may defy simple pigeonholing, it is of course necessary to attempt some classification (Fig. 1). Along with apoptosis another main type of eukaryotic cell death is autophagy (reviewed in ref. 16). As a recycling system for cellular components, autophagy can be used to prevent premature cell death. However, on a grander scale, this system can be used to enzymatically degrade cells to cause PCD; for example, release of vacuolar hydrolases into the cytosol of plant cells leads to the rapid breakdown of cellular components (reviewed in ref. 16).
Figure 1.
Cell Death Pathways in Plants and Animals. The broad spectrum of eukaryotic cell death pathways contain many shared and overlapping features, making simple definitions difficult. However, for the purposes of this article, the key features of each pathway are outlined below. (A) Apoptosis. In this animal-specific PCD pathway, the accumulation of intrinsic or extrinsic death signals leads to a number of distinct morphological changes, including an increase in mitochondrial division, nuclear and cytoplasmic condensation, and the formation of apoptotic bodies containing damaged cellular components. These apoptotic bodies are released into the extracellular matrix, where they are engulfed and degraded by phagocytosis. (B) Autophagy. Occurring in plants and animals, autophagy is an intracellular recycling process involving lysosomes or plant vacuoles. Upregulation of autophagy can enzymatically degrade cells, resulting in PCD. Induction of PCD using this pathway begins with the mitochondrial morphology transition (MMT) and DNA damage. This leads to the induction of autophagy, initially as a protective mechanism. Further progression of autophagy results in the engulfment of damaged cell components in the vacuole/lysosome, or rupture of the vacuole membrane, leading to degradation and PCD. (C) Classical plant PCD. This PCD pathway is typically defined by cell morphology, including the MMT, condensation of the plasma membrane from the cell wall and nuclear condensation.
As a self-contained, intracellular PCD process (cf. apoptosis), autophagy is a major (if not the major) pathway for plant cell death. However in the case of autophagy and other death programmes that may exist, a thorough, plant-focused evaluation of terminology is required, so that progress in the plant cell death field is not hampered by the strictures of another discipline.
Abbreviations
- PCD
programmed cell death
- ROS
reactive oxygen species
- GFP
green fluorescent protein
- UV-C
ultraviolet light-C
- MMT
mitochondrial morphology transition
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5678
References
- 1.Frank S, Gaume B, Bergmann Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 2.Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- 3.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 4.Logan DC, Leaver CJ. Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J Exp Bot. 2000;51:865–871. [PubMed] [Google Scholar]
- 5.Scott I, Logan DC. Mitochondrial morphology transition is an early indicator of subsequent cell death in Arabidopsis. New Phytol. 2008;177:90–101. doi: 10.1111/j.1469-8137.2007.02255.x. [DOI] [PubMed] [Google Scholar]
- 6.Gao C, Xing D, Li L, Zhang L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta. 2007 doi: 10.1007/s00425-007-0654-4. [DOI] [PubMed] [Google Scholar]
- 7.Yao N, Eisfelder BJ, Marvin J, Greenberg JT. The mitochondrion—an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J. 2004;40:596–610. doi: 10.1111/j.1365-313X.2004.02239.x. [DOI] [PubMed] [Google Scholar]
- 8.Yao N, Greenberg JT. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell. 2006;18:397–411. doi: 10.1105/tpc.105.036251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zottini M, Barizza E, Bastianelli F, Carimi F, Lo Schiavo F. Growth and senescence of Medicago truncatula cultured cells are associated with characteristic mitochondrial morphology. New Phytol. 2006;172:239–247. doi: 10.1111/j.1469-8137.2006.01830.x. [DOI] [PubMed] [Google Scholar]
- 10.Scott I, Tobin AK, Logan DC. BIGYIN, an orthologue of human and yeast FIS1 genes functions in the control of mitochondrial size and number in Arabidopsis thaliana. J Exp Bot. 2006;57:1275–1280. doi: 10.1093/jxb/erj096. [DOI] [PubMed] [Google Scholar]
- 11.Logan DC. The mitochondrial compartment. J Exp Bot. 2006;57:1225–1243. doi: 10.1093/jxb/erj151. [DOI] [PubMed] [Google Scholar]
- 12.Diamond M, McCabe PF. The mitochondrion and plant programmed cell death. In: Logan DC, editor. Plant Mitochondria. Oxford: Blackwell Publishing; 2007. [Google Scholar]
- 13.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan DC. Having a swell time—mitochondrial morphology and plant cell death programmes. J Microsc. 2008 doi: 10.1111/j.1365-2818.2008.02037.x. In press. [DOI] [PubMed] [Google Scholar]
- 16.van Doorn WG, Woltering EJ. Many ways to exit? Cell death categories in plants. Trends Plant Sci. 2005;10:117–122. doi: 10.1016/j.tplants.2005.01.006. [DOI] [PubMed] [Google Scholar]