Abstract
A common fate of post-mitotic interphase plant nuclei is morphological differentiation into an array of shapes and sizes. Development of nuclear morphology occurs in a cell-specific manner and is influenced by cell shape and nuclear DNA content. The LINC (LITTLE NUCLEI) proteins are plant-specific nuclear coiled-coil proteins that appear to couple nuclear development to cellular (shape) and nuclear (DNA content) cues. linc mutations cause a variety of defects, including smaller more spherical nuclei and whole-plant dwarfing. Supplementing our previous results, we constructed transgenic plants expressing LINC1-GFP from the native promoter and found that LINC1 is predominantly expressed in proliferating tissues. Moreover, LINC1-GFP signal was found to be concentrated at the nuclear periphery. These results suggest that LINC1 plays an important structural role at an early stage in nuclear development.
Key words: nuclear morphology, coiled-coil, nuclear envelope, Arabidopsis thaliana, LINC, lamin, nuclear architecture
The nucleus is a morphologically plastic organelle. In plants, the interphase nucleus is translucent and difficult to image by traditional light microscopy in unfixed and unstained tissue. Consequently, it was not until the Galbraith laboratory used a nuclear-localized GFP reporter molecule that a description of plant nuclear morphology dynamics and variation in living plant tissues was possible.1 These authors observed an assortment of nuclear shapes (from a variety of tissues) that fell into three general categories: spherical, spindle- shaped and cylindrical.1,2 Much of the morphological variation occurs post-mitotically when nuclei increase in size due to endoreduplication and differentiate in shape according to cell type.3–5 Examples of this differentiation are observed in anisotropic root hairs, which contain large elongated spindle-shaped nuclei.2 These nuclei contrast with the small spherical nuclei found within ovoid guard cells and isotropic meristematic tissues. However, beyond these descriptions little is known about the molecular determinants or cues that regulate nuclear morphology in plants.
The nucleus is an organelle surrounded by two continuous bi-layer membranes, the outer and inner nuclear envelopes. In mammals, coiled-coil type V intermediate filament proteins called lamins polymerize under the inner nuclear envelope forming a structural meshwork.6,7 In addition to providing structural support for the nucleus, lamins are important for the peripheral localization of a host of proteins, including nuclear pore complexes and proteins that anchor the nucleus to the cytoskeleton.8 Plants genomes do not encode lamins, yet a number of predicted nuclear coiled-coil proteins of unknown function were identified by bioinformatics.9
To gain a better understanding of nuclear morphology in plants we initiated a reverse genetics study on group of four long coiled-coil proteins in Arabidopsis thaliana that we called the LINC (LITTLE NUCLEI) genes. A plant homolog of the LINC proteins was originally identified in Daucus carota protoplasts and called NMCP1 (nuclear matrix constituent protein 1).10 This protein localizes to the nuclear periphery in D. carota protoplasts, leading to the hypothesis that NMCP1 functions as a lamin-like analog in plants.10,12
Plant harboring T-DNA insertional mutations at LINC1 (linc1-1), LINC2 (linc2-1), LINC3 (linc3-1, SALK_099283) and LINC4 (linc4-1, SALK_079296) were examined and found to exhibit no obvious phenotypic abnormalities. Because we anticipated redundancy within the LINC gene family, we constructed double and triple mutants and observed a range of whole-plant dwarfing defects for several of these linc mutant combinations. At the outset we limited ourselves to the dwarfed linc1-1 linc2-1 mutant. First, we examined fixed nuclei from adult rosette leaves and found that linc1-1 linc2-1 mutants have drastically smaller nuclei (∼20% of wild type).13 Furthermore, despite having normal whole-plant morphology, linc1-1 and linc2-1 single mutants also had reduced nuclear sizes (∼50% of wild type). During these analyses we noticed that the nuclei of the linc mutants, especially the linc1-1 linc2-1 double mutant, appeared more homogenous in shape than wild type nuclei. To quantify this feature we estimated the circularity index (4πA/P2 , A = area and P = perimeter) and found that on average linc1-1 and linc1-1 linc2-1 nuclei were significantly more spherical than wild type nuclei.13 These results indicated that both LINC1 and LINC2 are required to achieve wild type nuclear size and that LINC1 is also required to achieve a differentiated nuclear shape.13
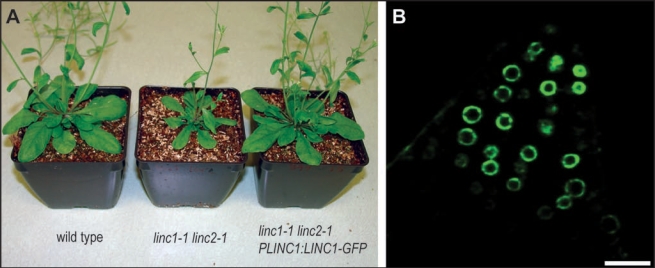
Our original paper described sub-cellular localization studies using YFP fusion proteins expressed from the Cauliflower Mosaic Virus 35S constitutive promoter.13 Consequently, we repeated these studies for LINC1 using its native promoter. A genomic clone of LINC1 including the upstream, intergenic regulatory sequence (∼2.5 kilo bases) was fused with the coding sequence of GFP, producing PLINC1:LINC1-GFP.14 Wild type and linc1-1 linc2-1 plants were transformed with the PLINC1:LINC1-GFP construct using the floral dip method and T1 transformants were selected on soil with basta herbicide.15 We determined whether PLINC1:LINC1-GFP is functional by asking whether the transgene could complement the linc1-1 mutation and rescue the synthetic linc1-1 linc2-1 whole-plant dwarfing defect. A representative transgenic (T2) linc1-1 linc2-1 plant transformed with PLINC1:LINC1-GFP construct is shown in Figure 1A. This transgenic plant displayed a wild type stature and size (Fig. 1A) indicating that expression of the LINC1-GFP fusion protein from its native promoter complements the linc1-1 loss-of-function mutation. Next we examined roots of T2 seedlings by fluorescence microscopy and found that the LINC1-GFP signal was high in proliferating meristematic tissues, such as the root tip, but was not detected in differentiated root tissues, such as mature root hairs and epidermal cells.
Figure 1.
PLINC1:LINC1-GFP complemented linc1-1 and LINC1-GFP was concentrated at the nuclear periphery. (A) From left to right are a wild type plant, a linc1-1 linc2-1 double mutant, and a transgenic linc1-1 linc2-1 plant transformed with PLINC1:LINC1-GFP. (B) is a single confocal section through a budding lateral root from a one-week-old linc1-1 linc2-1 transgenic PLINC1:LINC1-GFP seedling. Bar equals 20 micrometers.
We determined the subcellular localization of LINC1-GFP in roots from week-old seedlings using laser scanning confocal microscopy. We found that LINC1-GFP accumulated within nuclei and was highly concentrated at the nuclear periphery. Figure 1B shows a single confocal section through a budding lateral root illustrating the localization of LINC1-GFP at the nuclear envelope. We note that LINC1-GFP signal rapidly tapered off moving from the lateral root into differentiated root cortical tissue. The subcellular localization of LINC1-GFP expressed from its native promoter was indistinguishable from our previous results for LINC1-YFP expressed using the constitutive 35S promoter.
Despite the well-documented occurrence of nuclear morphological variation in plants, little is known about the molecular determinants involved in this process. The LINC proteins provide an attractive entrée into this phenomenon. Because the LINC proteins are predicted to dimerize through a long coiled-coiled domain, and linc mutations cause several nucleus-specific morphological defects, it is possible that these proteins play a structural role within the nucleus. It is intriguing that the nuclear phenotype of the linc1-1 linc2-1 mutant is most apparent in differentiated nuclei, tissues in which the LINC1 promoter is not active (Fig. 1B). The specific LINC1 promoter activity in proliferating tissue suggests that LINC1 functions at a key differentiation step following nuclear formation. One possibility is that linc1-1 mutant nuclei fail to establish appropriate connections with the cytoskeleton and are therefore unable to respond to cellular cues during morphogenesis. Alternatively, it is conceivable that the LINC proteins facilitate nuclear envelope expansion by regulating the addition of membrane components. More work is needed to elucidate the mechanism of LINC action in nuclear differentiation.
Acknowledgements
We thank the entire Richards' lab for useful discussion. This work was supported by a grant from the National Science Foundation (to E.J.R., MCB-0548597)
Abbreviations
- LINC
little nuclei
- NMCP1
nuclear matrix constituent protein 1
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5682
References
- 1.Chytilova E, Macas J, Sliwinska E, Rafelski SM, Lambert GM, Galbraith DW. Nuclear dynamics in Arabidopsis thaliana. Mol Biol Cell. 2000;11:2733–2741. doi: 10.1091/mbc.11.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chytilova EVA, Macas J, Galbraith DW. Green fluorescent protein targeted to the nucleus, transgenic phenotype useful for studies in plant biology. Ann Bot. 1999;83:645–654. %R 10.1006/anbo.1999.0866. [Google Scholar]
- 3.Galbraith DW, Harkins KR, Knapp S. Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol. 1991;96:985–989. doi: 10.1104/pp.96.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimoto Shirasu K, Roberts K. “Big it up” :endoreduplication and cell-size control in plants. Curr Opin Plant Biol. 2003;6:544–553. doi: 10.1016/j.pbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Fang Y, Hearn S, Spector DL. Tissue-specific expression and dynamic organization of SR splicing factors in Arabidopsis. Mol Biol Cell. 2004;15:2664–2673. doi: 10.1091/mbc.E04-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 2002;16:533–547. doi: 10.1101/gad.960502. [DOI] [PubMed] [Google Scholar]
- 7.Gruenbaum Y, Goldman RD, Meyuhas R, Mills E, Margalit A, Fridkin A, Dayani Y, Prokocimer M, Enosh A. The nuclear lamina and its functions in the nucleus. Int Rev Cytol. 2003;226:1–62. doi: 10.1016/s0074-7696(03)01001-5. [DOI] [PubMed] [Google Scholar]
- 8.Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol. 2005;6:21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- 9.Rose A, Manikantan S, Schraegle SJ, Maloy MA, Stahlberg EA, Meier I. Genome-wide identification of Arabidopsis coiled-coil proteins and establishment of the ARABI-COIL database. Plant Physiol. 2004;134:927–939. doi: 10.1104/pp.103.035626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuda K, Xu ZJ, Takahashi S, Ito A, Ono M, Nomura K, Inoue M. Peripheral framework of carrot cell nucleus contains a novel protein predicted to exhibit a long alpha-helical domain. Exp Cell Res. 1997;232:173–181. doi: 10.1006/excr.1997.3531. [DOI] [PubMed] [Google Scholar]
- 11.Rose A, Patel S, Meier I. The plant nuclear envelope. Planta. 2004;218:327–336. doi: 10.1007/s00425-003-1132-2. [DOI] [PubMed] [Google Scholar]
- 12.Meier I. Composition of the plant nuclear envelope: theme and variations. J Exp Bot. 2007;58:27–34. doi: 10.1093/jxb/erl009. [DOI] [PubMed] [Google Scholar]
- 13.Dittmer TA, Stacey NJ, Sugimoto-Shirasu K, Richards EJ. LITTLE NUCLEI genes affecting nuclear morphology in Arabidopsis thaliana. Plant Cell. 2007;19:2793–2803. doi: 10.1105/tpc.107.053231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1988;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]