Abstract
Electrical signaling, short-term memory and rapid closure of the carnivorous plant Dionaea muscipula Ellis (Venus flytrap) have been attracting the attention of researchers since the XIX century. We found that the electrical stimulus between a midrib and a lobe closes the Venus flytrap upper leaf without mechanical stimulation of trigger hairs. The closing time of Venus flytrap by electrical stimulation is the same as mechanically induced closing. Transmission of a single electrical charge between a lobe and the midrib causes closure of the trap and induces an electrical signal propagating between both lobes and midrib. The Venus flytrap can accumulate small subthreshold charges, and when the threshold value is reached, the trap closes. Repeated application of smaller charges demonstrates the summation of stimuli. The cumulative character of electrical stimuli points to the existence of short-term electrical memory in the Venus flytrap.
Key words: plant memory, electrophysiology, electrical signaling, venus flytrap, Dionaea muscipula ellis
Plants are capable of intelligent responses to complex environmental signals.1–27 Signaling and memory play fundamental roles in plant responses. The existence of different forms of plant memory is well known.1–22 Depending on the duration of memory retention, there are three types of memory in plants: sensory memory, short term memory and long term memory. A few examples of studies involving plant memory are: transgeneration memory of stress,1,6,10 immunological memory of tobacco plants22 and mountain birches,18 storage and recall functions in seedlings,9 chromatin remodelling in plant development,4,19 vernalization and epigenetic memory of winter,12,13 induced resistance and susceptibility to herbivory,2 memory response in ABA-entrained plants,6 memory of stimulus,16,17 and systematic acquired resistance in plants exposed to a pathogen.22 Cellular memory is an example of long term memory and is a long-term maintenance of a particular pattern of gene expression. Chromatin dynamics including histone modification, histone replacement and chromatin remodeling play key roles in cellular memory.4 Plants are intelligent organisms and capable of functions such as learning, individuality, plasticity and memory.5 There are a few mathematical models of plant learning and memory.14,15 Some plants exhibit clues of an electrical memory as well.
We found that Venus flytrap has a short term electrical memory20,21 Rapid closure of the carnivorous plant Dionaea muscipula Ellis (Venus flytrap) has been attracting the attention of researchers and as a result its mechanism has been widely investigated. When an insect touches the trigger hairs, these mechanosensors generate an electrical signal that acts as an action potential, which activates the trap closing. Macfarlane23 found that two mechanical stimuli required for the trap closing should be applied within an interval from 0.75 s to 20 s. Brown and Sharp24 found that at high temperature of 35–40°C usually only one mechanical stimulus is required.
The inducement of non-excitability after excitation and the summation of subthreshold irritations were developed in the vegetative and animal kingdoms in protoplasmic structures prior to morphological differentiation of nervous tissues. These protoplasmic structures merged into the organs of a nervous system and adjusted the interfacing of the organism with the environment. Some neuromotoric components include acetylcholine neurotransmitters, cellular messenger calmodulin, cellular motors actin and myosin, voltage-gated channels, and sensors for touch, light, gravity and temperature.25–27 Although this nerve-like cellular equipment has not reached the same great complexity as in animal nerves, a simple neural network has been formed within the plasma membrane of a phloem or plasmodesmata enabling it to communicate efficiently over long distances.5,26,27 The reason why plants have developed pathways for electrical signal transmission most probably lies in the necessity to respond rapidly to environmental stress factors. Different environmental stimuli evoke specific responses in living cells, which have the capacity to transmit a signal to the responding region. In contrast to chemical signals such as hormones, electrical signals are able to rapidly transmit information over long distances.27 Electrical potentials have been measured at the tissue and whole plant levels.26
Using our new charge injection method,20 it was evident that the application of an electrical stimulus between the midrib (positive potential) and a lobe (negative potential) causes Venus flytrap to close the trap without any mechanical stimulation. The average stimulation pulse voltage sufficient for rapid closure of the Venus flytrap was 1.50 V (standard deviation is 0.01 V, n = 50) for 1 s. The inverted polarity pulse with negative voltage applied to the midrib did not close the plant. Applying impulses in the same voltage range with different polarities for pulses of up to 100 s did not open the plant. It was found that energy for trap closure is generated by ATP hydrolysis. ATP is used by the motor cells for a fast transport of protons. The amount of ATP drops from 950 µM per midrib before mechanical stimulation to 650 µM per midrib after stimulation and closure.28 However, it is not clear if electrical stimulation triggers closing process in the motor cells, or contributes energy to the closing action.
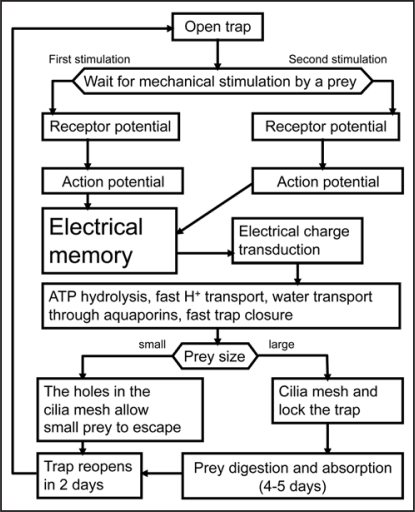
The action potential delivers sufficient electrical charge to the midrib,21 which can activate the osmotic motor. To check this hypothesis, we measured effects of transmitted charge from the charged capacitors between the lobe and the midrib of Venus flytrap. Transmission of a single electrical charge (mean 13.63 µC, median 14.00 µC, std. dev. 1.51 µC, n = 41) causes trap closure and induces an electrical signal propagating between the lobes and the midrib. The electrical signal in the lobes was not an action potential, because its amplitude depended on the applied voltage from the charged capacitor. Charge induced closing of a trap plant can be repeated 2–3 times on the same Venus flytrap plant after reopening. Transmission of a single electrical charge (mean 13.63 µC, median 14.00 µC, std. dev. 1.51 µC, n = 41) causes the trap to close and induces an electrical signal that propagates between the lobe and the midrib. Figure 1 illustrates that the Venus flytrap can accumulate small charges, and when the threshold value is reached, the trap closes. A summation of stimuli is demonstrated through the repetitive application of smaller charges. If we apply two or more consecutive injections of electrical charge within a period of less than 50 s, the trap will close when a total of 14 µC charge is reached.
Figure 1.
Mechanism of the Dionaea trap closure.
Repeated application of smaller charges demonstrates a summation of stimuli. If we apply two or more injections of electrical charges within a period of less then 20 s, the Venus flytrap upper leaf closes as soon as the total of 14 µC charge is transmitted. Similar phenomenon was reported by Czaja,29 who determined the intensity of threshold stimuli to be 2.4 µC for a closing electrostimulation of another carnivorous plant Aldrovanda vesiculosa, and 0.91 µC for an opening electrostimulation. Our attempts to open the Venus flytrap upper leaf by changing polarity of injected charge and increasing the charge from 14 µC to 100 µC were not successful. Usually, the trap opens a few days after closing in the same way as after mechanically stimulated closing.
Previous work by Brown and Sharp24 indicated that electrical shock between lower and upper leaves can cause the Venus flytrap to close, but in their article, the amplitude and polarity of applied voltage, charge and electrical current were not reported. The trap did not close when we applied the same electrostimulation between the upper and lower leaves as we applied between a midrib and a lobe, even when the injected charge was increased from 14 µC to 750 µC. It is probable that the electroshock induced by Brown and Sharp24 had a very high voltage or electrical current.
It is common knowledge that the leaves of the Venus flytrap actively employ turgor pressure and hydrodynamic flow for fast movement and catching insects. In these processes the upper and lower surfaces of the leaf behave quite differently. During the trap closing, the loss of turgor by parenchyma lying beneath the upper epidermis, accompanied by the active expansion of the tissues of the lower layers of parenchyma near the under epidermis, closes the trap. The cells on the inner face of the trap jettison their cargo of water, shrink and allow the trap lobe to fold over. The cells of the lower epidermis expand rapidly, folding the trap lobe over. These anatomical features constitute the basis of the new hydroelastic curvature model.20
In terms of electrophysiology, Venus flytrap responses can be considered in three stages: (i) stimulus perception, (ii) signal transmission and (iii) induction of response (Fig. 1).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5684
References
- 1.Molinier J, Ries G, Zipfel C, Hohn B. Transgeneration memory of stress in plants. Nature. 2006;442:1046–1049. doi: 10.1038/nature05022. [DOI] [PubMed] [Google Scholar]
- 2.Karban R, Niiho C. Induced resistance and susceptibility to herbivory: plant memory and altered plant development. Ecology. 1995;76:1220–1225. [Google Scholar]
- 3.Baldwin IT, Schmelz EA. Immunological “memory” in the induced accumulation of nicotine in wild tobacco. Ecology. 1996;77:236–246. [Google Scholar]
- 4.Reyes JC, Hennig L, Gruissem W. Chromatin-remodeling and memory factors. New regulators of plant development. Plant Physiol. 2002;130:1090–1101. doi: 10.1104/pp.006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trewavas A. Aspects of plant intelligence. Annals Botany. 2003;92:1–20. doi: 10.1093/aob/mcg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goh CH, Nam HG, Park YS. Stress memory in plants: a negative regulation of stomatal response and transient induction of rd22 gene to light in abscisic acid-entrained Arabidopsis plants. Plant J. 2003;36:240–255. doi: 10.1046/j.1365-313x.2003.01872.x. [DOI] [PubMed] [Google Scholar]
- 7.Nick P, Schafer E. Spatial memory during the tropism of maize (Zea mays L.) coleoptiles. Planta. 1988;175:380–388. doi: 10.1007/BF00396344. [DOI] [PubMed] [Google Scholar]
- 8.Nick P, Sailer K, Schafer E. On the relation between photo- and gravitropically induced spatial memory in maize coleoptiles. Planta. 1990;181:385–392. doi: 10.1007/BF00195892. [DOI] [PubMed] [Google Scholar]
- 9.Thellier M, Sceller LL, Norris V, Verdus MC, Ripoll C. Long-distance transport, storage and recall of morphogenetic information in plants. The existence of a sort of primitive plant “memory”. CR Acad Sci Paris. 2000;323:81–91. doi: 10.1016/s0764-4469(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 10.Bruce TJA, Matthes MC, Napier J, Pickett JA. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007;173:603–608. [Google Scholar]
- 11.Soja G, Eid M, Gangl H, Redl H. Ozone sensitivity of grapevine (Vitis vinifera L.): evidence for a memory effect in a perennial crop plant? Phyton Ann Rei Bot. 1997;37:265–270. [Google Scholar]
- 12.Sung S, Amasino RM. Vernalisation and epigenetics: how plants remember winter. Curr Opin Plant Biol. 2004;7:4–10. doi: 10.1016/j.pbi.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Sung S, Amasino RM. Molecular genetic study of the memory of winter. J Exp Bot. 2006;57:3369–3377. doi: 10.1093/jxb/erl105. [DOI] [PubMed] [Google Scholar]
- 14.Demongeot J, Thomas R, Thellier M. A mathematical model for storage and recall functions in plants. C R Acad Sci. 2000;323:93–97. doi: 10.1016/s0764-4469(00)00103-7. [DOI] [PubMed] [Google Scholar]
- 15.Bose I, Rajesh K. Simple models of plant learning and memory. Physica Scripta. 2003;106:9–12. [Google Scholar]
- 16.Ueda M, Nakamura Y, Okada M. Endogeneous factors involved in the regulation of movement and “memory” in plants. Pure Appl Chem. 2007;79:519–527. [Google Scholar]
- 17.Ueda M, Nakamura Y. Metabolites involved in plant movement and “memory”: nyctinasy of legumes and trap movement in the Venus flytrap. Natural Product Reports. 2006;23:548–557. doi: 10.1039/b515708k. [DOI] [PubMed] [Google Scholar]
- 18.Ruuhola T, Salminen JP, Haviola S, Yang S, Rantala MJ. Immunological memory of mountain birches: effects of phenolics on performance of the autumnal moth depend on herbivory history of trees. J Chem Ecology. 2007;33:1160–1176. doi: 10.1007/s10886-007-9308-z. [DOI] [PubMed] [Google Scholar]
- 19.Goodrich J, Tweedie S. Remembrance of things past: chromatin remodeling in plant development. Annual Review Cell Developmental Biology. 2002;18:707–746. doi: 10.1146/annurev.cellbio.18.040202.114836. [DOI] [PubMed] [Google Scholar]
- 20.Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol. 2008;146:696–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkov AG, Adesina T, Jovanov E. Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signal Behavior. 2007;2:139–145. doi: 10.4161/psb.2.3.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conrath U. Systemic acquired resistance. Plant Signal Behavior. 2006;1:179–184. doi: 10.4161/psb.1.4.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macfarlane JM. Contributions to the history of Dionaea muscipula Ellis. Contrib Bot Lab Penna. 1892;1:7–44. [Google Scholar]
- 24.Brown WH, Sharp LW. The closing response in Dionaea. Bot Gaz. 1910;49:290–302. [Google Scholar]
- 25.Roshchina VV. Neurotransmitters in Plant Life. Enfield: Science Publ; 2001. [Google Scholar]
- 26.Volkov AG, editor. Plant Electrophysiology. Berlin: Springer; 2006. [Google Scholar]
- 27.Volkov AG. Green plants: Electrochemical interfaces. J Electroanal Chem. 2000;483:150–156. [Google Scholar]
- 28.Jaffe MJ. The role of ATP in mechanically stimulated rapid closure of the Venus's flytrap. Plant Physiol. 1973;51:17–18. doi: 10.1104/pp.51.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czaja ATh. Reizphysiologische Untersuchungen an Aldrovandia vesiculosa L. Arch Gesamte Physiologie Menschen Tiere. 1924;206:635–658. [Google Scholar]