Abstract
In Medicago truncatula high rates of somatic embryo formation can be induced in the Jemalong genotype 2HA by application of the hormones auxin and cytokinin. Biosynthesis of the stress-related hormone ethylene is also necessary for somatic embryogenesis (SE) and is most likely a response to wounding and the presence of auxin in the medium. We have demonstrated that expression of a gene designated Mt SOMATIC EMBRYO RELATED FACTOR 1 (MtSERF1) induced by ethylene, in the presence of auxin plus cytokinin, is essential for SE. The promoter region of this transcription factor, a member of the ERF sub-family of the AP2/ERF super family, contains putative binding sites relating to auxin and cytokinin in addition to ethylene. An additional finding was the presence of WUSCHEL (WUS) binding sites in the MtSERF1 promoter region, which is discussed. Here we also discuss the Medicago data in the context of embryogenesis studies in Arabidopsis and suggest that MtSERF1 has a key developmental role, possibly in conjunction with WUS, in regulating downstream genes required for the initiation of SE.
Key words: somatic embryogenesis, MtSERF1, ethylene, auxin, cytokinin, Medicago truncatula
Attempts to decipher the mechanisms underlying the induction of somatic embryogenesis (SE) have resulted in identification of several genes and transcription factors whose overexpression has been shown to effectively enhances the regenerative competence of plant cells for somatic embryogenesis, including SERK1, LEC1, LEC2, BBM, AGL15 and WUSCHEL.1–6 Nonetheless, the precise molecular mechanisms involved in the induction of SE from cultured tissue are not well understood.
In Medicago truncatula high rates of somatic embryo formation can be induced in the Jemalong genotype 2HA7 by application of the hormones auxin and cytokinin.8 In addition to the application of hormones to induce somatic embryogenesis there is the stress component, induced by the excision and culture of the explant, to consider.9 In M. truncatula there are many stress-related proteins associated with SE.10 A number of these proteins are differentially expressed between 2HA and wild-type Jemalong.11 The synthesis of the stress-related hormone ethylene can be rapidly evoked in response to a variety of biotic and abiotic stresses including wounding.12,13 The wound effect would most likely be augmented by the presence of auxin in the medium.14
We have recently demonstrated an important role for an AP2/ERF transcription factor designated the MEDICAGO TRUNCATULA SOMATIC EMBRYO RELATED FACTOR 1 (MtSERF1) in somatic embryogenesis in the model legume M. truncatula.15 The idea that MtSERF1 is embryo specific and required for SE in M. truncatula is supported by several lines of evidence. Its expression is increased during the culture period and reaches a peak at approximately 3 weeks. In our leaf explant culture this is the time embryos are forming. In wild-type Jemalong where SE is rare there is no increase in MtSERF1 expression. Furthermore, in situ mRNA localization analysis reveals that its transcripts are preferentially localized in embryos and not in callus cells. Finally, RNAi silencing of MtSERF1 results in virtually total suppression of somatic embryo formation.15
In our study15 we demonstrated that ethylene was required for the transcription of MtSERF1. The importance of ethylene for its upregulation is highlighted by the following findings: first, qRT-PCR experiments showed that when either the inhibitor of ethylene biosynthesis or perception was added to the medium MtSERF1 was downregulated; second, in silico analysis of its promoter region revealed the presence of three ethylene regulatory regions; and third, no signal was detected by in situ RNA localization when explants cultured on medium containing the inhibitor of ethylene perception were hybridized with either antisense or sense MtSERF1 RNA probes.15 As in our previous studies auxin and cytokinin were present in the medium to induce SE.16 Both auxin and cytokinin are necessary for MtSERF1 transcription as qRT-PCR experiments reveal that when either auxin or cytokinin is absent MtSERF1 is downregulated. Furthermore the MtSERF1 promoter region contains putative binding sites relating to auxin and cytokinin in addition to ethylene. These data overall support the idea that the stress hormone ethylene produced in response to the stress of the explant excision and culture interacts with the growth and differentiation hormones auxin and cytokinin to promote SE. MtSERF1 transcription may act as a nexus for auxin, cytokinin and ethylene action to promote SE. It has been suggested that transcription factors interconnect different hormone pathways and play an important role in hormone signal transduction.17 Furthermore, it is well established that transcription factors mediate key effects of hormones in development.The finding of a relationship between an ERF subfamily gene and the formation of somatic embryos in vitro is consistent with an emerging picture of the involvement of ERF proteins in developmental processes studied in vitro. The ENHANCER OF SHOOT REGENERATION 1 and 2 (ESR1 and ESR2)18,19 and RAP2.6L20 have a role in shoot regeneration in Arabidopsis. BABY BOOM (BBM), a member of the AP2 family possessing two repeated AP2/ERF domains,4,21 has been shown to induce somatic embryogenesis when overexpressed in Arabidopsis and Brassica napus.4 Recently a gene called ERF REQUIRED FOR NODULATION (ERN), has been identified in Medicago truncatula.22 Furthermore ethylene and PLETHORA, other member of the AP2/ERF superfamily are involved in stem cell maintenance in the root apex.23,24 Although, all these genes were studied in the context of developmental roles, most of the ERF proteins have been studied in relation to biotic and abiotic stress.25 Therefore, it appears that the AP2/ERF superfamily has a mix of transcription factors involved in growth and development as well as abiotic and biotic stress. This may relate to the need to link development and stress in the evolution of sessile plants.
Another interesting finding from this work is the presence of two WUSCHEL binding sites in the promoter region of MtSERF1, which raises the possibility that there is a link between WUS and MtSERF1. This is further supported by the fact that both genes are found to similarly localize in the embryo of M. truncatula and WUS (the M. truncatula homolog) expression is maximal prior to MtSERF1 expression (our unpublished data). It has been well appreciated that WUS plays a dominant role in regulating the stem cell population in shoot and flower meristems;26,27 however, WUS has also been shown to have the ability to be a somatic embryo organiser in Arabidopsis.6 The almost total suppression of the embryogenic capacity of MtSERF1 knockdown suggests a failure to promote or maintain an embryonic stem cell fate in the cultured leaf explants. It is therefore possible that MtSERF1 functions downstream of WUS as a positive regulator in promoting or maintaining an embryonic stem cell fate.
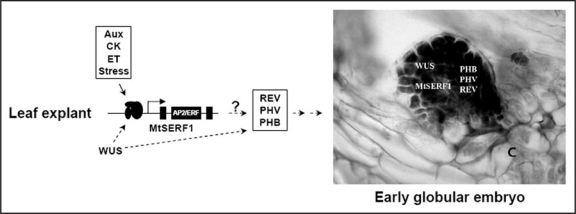
The downstream targets of the MtSERF1 transcription factor are not known. One possibility is that MtSERF1 interacts with HD-Zip III genes involved in the determination of cell fate and embryonic patterning, including PHABULOSA (PHB), PHAVOLUTA (PHV), and REVOLUTA (REV), as these genes are also expressed throughout the early globular embryo in Arabidopsis28,29 and are also required for normal shoot apical meristem (SAM) development. The interaction between one of these genes with a member of the ERF subfamily has been demonstrated recently.30 Chandler and co-workers demonstrated that a heterodimeric complex between ESR1 or ESR2 and PHV or related HD-ZIP III proteins is necessary to control the transcription of target genes required for embryo patterning and normal cotyledon development.30 Therefore, it is also possible that cross-coupling between MtSERF1 and one of these genes is needed in mediating the induction of SE. Further, there is also the possibility that MtSERF1 is involved in a regulatory interaction with WUS and these HD-ZIP III proteins. Sieber et al.,31 have demonstrated that WUS and PHB regulate processes during the transition from proximal-distal to adaxial-abaxial ovule development. The overlapping expression of all these genes at the early globular-stage embryo make this idea an attractive hypothesis (Fig. 1).
Figure 1.
The overlapping expression of MtSERF1, WUS and HD-Zip III genes (PHB, PHV, REV) in the early globular-stage embryo and the proposed interaction between these genes leading to the induction of SE. AP2/ERF, the AP/2/ERF domain; Aux, auxin; C, callus tissue; CK, cytokinin; ET, ethylene.
Understanding precisely how MtSERF1 is regulated by different hormone and stress factors and how MtSERF1 interacts with other genes or transcription factors involved in the promotion and maintenance of stem cells and embryo patterning should provide a useful contribution to understanding how a single somatic cell can become a whole plant.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6049
References
- 1.Hecht V, Vielle Calzada JP, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U, de Vries SC. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001;127:803–816. [PMC free article] [PubMed] [Google Scholar]
- 2.Lotan T, Ohto M, Yee KM, West MAL, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93:1195–1205. doi: 10.1016/s0092-8674(00)81463-4. [DOI] [PubMed] [Google Scholar]
- 3.Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA. 2001;98:11806–11811. doi: 10.1073/pnas.201413498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang LM, Hattori J, Liu CM, van Lammeren AAM, Miki BLA, Custers JBM, van Lookeren Campagne MM. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14:1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE. Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-like 15. Plant Physiol. 2003;133:653–663. doi: 10.1104/pp.103.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo J, Niu QW, Frugis G, Chua NH. The WUSCHEL gene promotes vegetative-toembryonic transition in Arabidopsis. Plant J. 2002;30:349–359. doi: 10.1046/j.1365-313x.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 7.Rose RJ, Nolan KE, Bicego L. The development of the highly regenerable seed line Jemalong 2HA for transformation of Medicago truncatula—implications for regenerability via somatic embryogenesis. J Plant Physiol. 1999;55:788–791. [Google Scholar]
- 8.Nolan KE, Irwanto RR, Rose RJ. Auxin upregulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol. 2003;133:218–230. doi: 10.1104/pp.103.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan KE, Saeed NA, Rose RJ. The stress kinase gene MtSK1 in Medicago truncatula with particular reference to somatic embryogenesis. Plant Cell Rep. 2006;25:711–722. doi: 10.1007/s00299-006-0135-4. [DOI] [PubMed] [Google Scholar]
- 10.Imin N, De Jong F, Mathesius U, Van Noorden G, Saeed NA, Wang XD, Rose RJ, Rolfe BG. Proteome reference maps of Medicago truncatula embryogenic cell cultures generated from single protoplasts. Proteomics. 2004;4:1883–1896. doi: 10.1002/pmic.200300803. [DOI] [PubMed] [Google Scholar]
- 11.Imin N, Nizamidin M, Daniher D, Nolan KE, Rose RJ, Rolfe BG. Proteomic analysis of somatic embryogenesis in Medicago truncatula. Explant cultures grown under 6-benzylaminopurine and 1-naphthaleneacetic acid treatments. Plant Physiol. 2005;137:1250–1260. doi: 10.1104/pp.104.055277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kende H, Zeevaart JAD. The five “classical” plant hormones. Plant Cell. 1997;9:1197–1210. doi: 10.1105/tpc.9.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang KL, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14:131–151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song YJ, Joo JH, Ryu HY, Lee JS, Bae YS, Nam KH. Reactive oxygen species mediate IAA-induced ethylene production in mungbean (Vigna radiata L.) hypocotyls. J Plant Biol. 2007:5018–5023. [Google Scholar]
- 15.Mantiri FR, Kurdyukov S, Lohar DP, Sharopova N, Saeed NA, Wang XD, VandenBosch KA, Rose RJ. The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiol. 2008;146:1622–1636. doi: 10.1104/pp.107.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolan KE, Rose RJ. Plant regeneration from cultured Medicago truncatula with particular reference to abscisic acid and light treatments. Aust J Bot. 1998;46:151–160. [Google Scholar]
- 17.Vogler H, Kuhlemeier C. Simple hormones but complex signalling. Curr Opin Plant Biol. 2003;6:51–56. doi: 10.1016/s1369-5266(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 18.Banno H, Ikeda Y, Niu QW, Chua NH. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell. 2001;13:2609–2618. doi: 10.1105/tpc.010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda Y, Banno H, Niu QW, Howell SH, Chua NH. The ENHANCER OF SHOOT REGENERATION 2 gene in Arabidopsis regulates CUP-SHAPED COTYLEDON 1 at the transcriptional level and controls cotyledon development. Plant Cell Physiol. 2006;47:1443–1456. doi: 10.1093/pcp/pcl023. [DOI] [PubMed] [Google Scholar]
- 20.Che P, Lall S, Nettleton D, Howell SH. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol. 2006;141:620–637. doi: 10.1104/pp.106.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140:411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton PH, Jakab J, Penmetsa RV, Starker CG, Doll J, Kalo P, Prabhu R, Marsh JF, Mitra RM, Kereszt A, Dudas B, VandenBosch K, Long SR, Cook DR, Kiss GB, Oldroyd GED. An ERF transcription factor in Medicago truncatula that is essential for nod factor signal transduction. Plant Cell. 2007;19:1221–1234. doi: 10.1105/tpc.106.048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh Y, Amasino R, Scheres B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Ortega Martinez O, Pernas M, Carol RJ, Dolan L. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science. 2007;317:507–510. doi: 10.1126/science.1143409. [DOI] [PubMed] [Google Scholar]
- 25.Riechmann JL, Meyerowitz EM. The AP2/EREBP family of plant transcription factors. Biol Chem. 1998;379:633–646. doi: 10.1515/bchm.1998.379.6.633. [DOI] [PubMed] [Google Scholar]
- 26.Laux T, Mayer KFX, Berger J, Jürgens G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development. 1996;122:87–96. doi: 10.1242/dev.122.1.87. [DOI] [PubMed] [Google Scholar]
- 27.Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–815. doi: 10.1016/s0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- 28.Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL. Radial patterning of Arabidopsis shoots by class IIIHD-ZIP and KANADI genes. Curr Biol. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 29.Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE. ClassIII homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005;17:61–76. doi: 10.1105/tpc.104.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandler JW, Cole M, Flier A, Grewe B, Werr W. The AP2 transcription factors DORNROSCHEN and DORNROSCHEN-LIKE redundantly control Arabidopsis embryo patterning via interaction with PHAVOLUTA. Development. 2007;134:1653–1662. doi: 10.1242/dev.001016. [DOI] [PubMed] [Google Scholar]
- 31.Sieber P, Gheyselinck J, Gross-Hardt R, Laux T, Grossniklaus U, Schneitz K. Pattern formation during early ovule development in Arabidopsis thaliana. Dev Biol. 2004;273:321–334. doi: 10.1016/j.ydbio.2004.05.037. [DOI] [PubMed] [Google Scholar]