Abstract
Seven transmembrane G-protein-coupled receptors (GPCRs) are commonly used by eukaryotes to sense extracellular signals to switch on cellular responses through the activation of cognate heterotrimeric G-proteins. In Arabidopsis thaliana, GCR2 has been proposed as a GPCR for the plant hormone abscisic acid. On the other hand, biochemical analysis demonstrates that the sole Arabidopsis heterotrimeric G-protein α subunit, GPA1, is in the activated state (GTP-bound) by default, suggesting that the heterotrimeric G-proteins may act without any GPCRs.
Key words: heterotrimeric G-proteins, GCR2, GPA1, G-protein-coupled receptor (GPCR), AtRGS1
Heterotrimeric G-Proteins are Conserved Molecular Switches
Signaling through the heterotrimeric G-proteins (G-proteins) is one of the most ancient and evolutionarily conserved mechanisms found in eukaryotes. As defined in metazoans and in yeasts, G-proteins act as molecular switches to couple the activation of a specific class of cell-surface receptors, seven transmembrane (7TM) G-protein-coupled receptors (GPCRs), to the intracellular signaling cascades leading to corresponding cellular responses. The heterotrimeric G-protein subunits are conserved in plants.1,2 Analyses of the loss-of-function alleles and gain-of-function overexpression lines of heterotrimeric G-protein subunits in Arabidopsis and rice suggested that G-proteins play important roles in hormonal signaling and stress responses, and regulate diverse processes of plant development.3 However, elucidation of a complete cascade of G-protein signaling is still lacking. In particular, the hunt for a bona fide plant GPCR has attracted much attention over the last 10 years. Recently, Liu et al., reported that Arabidopsis GCR2 is a GPCR for the plant hormone abscisic acid (ABA).4 On the other hand, Johnston et al., demonstrated that the sole Arabidopsis heterotrimeric G-protein α subunit (Gα), GPA1, is in the activated state (GTP-bound) by default,5 raising the possibility that the heterotrimeric G-proteins may act without any canonical GPCRs in Arabidopsis. These findings represent critical developments in the field of plant heterotrimeric G-proteins, and open up some fundamental questions about the heterotrimeric G-protein signaling mechanisms.
Is GCR2 a Bona Fide 7TM GPCR?
GCR2 was identified in a bioinformatic screen for 7TM proteins encoded by the Arabidopsis genome using the primitive Dense Alignment Surface and TMpred transmembrane segment prediction systems.4 Subsequently, it was shown that GCR2 physically interacts with GPA1, the sole heterotrimeric Gα in Arabidopsis. Furthermore, the physiologically active form of ABA, but not the inactive form of ABA, induces the dissociation of the GCR2-GPA1 complex. Because ABA specifically binds GCR2 at physiological concentrations with expected kinetics and stereospecificity, and putative loss-of-function alleles of GCR2 displayed insensitivity to ABA in all known major ABA responses including seed germination, early seedling development, stomatal movement and ABA-induced gene expression, it was concluded that GCR2 is an ABA-signaling GPCR.4 The significance of this finding was that it identified a long-awaited, the first plasma membrane receptor for ABA and it identified the first GPCR together with its ligand in plants.
However, the structure and functionality of GCR2 were immediately challenged by other independent studies.6–8 Several lines of evidence contradicted the notion that GCR2 is a GPCR for ABA. First, GCR2 was predicted not to be a 7TM protein in several robust transmembrane prediction systems.6–8 This is a critical result, because the 7TM domain is the structural hallmark of any classical GPCRs. The ease of solubilization of recombinant GCR2 proteins expressed in E.coli4 also disfavors the notion that GCR2 is an integral membrane protein. Second, GCR2 has significant sequence and predicted structural similarity to homologs of bacterial lanthionine synthetase (LanC) in diverse species. Homology modeling using the crystal structure of the Lactococcus lactis LanC protein, nisin cyclase (NisC),9 as template revealed that the core α-helices of NisC superimpose very well to those of the GCR2, and that the zinc-coordinating residues of NisC, important for cysteine cyclization, superimpose well with the corresponding residues of the GCR2 model, providing a structural argument that GCR2 is a member of the LanC protein family.6 No member of this protein family has been shown to be a transmembrane protein or a GPCR. Finally, the putative loss-of-function gcr2 mutants displayed hyposensitivity or wild-type response to ABA under different experimental conditions in seed germination, early seedling development and ABA-induced gene expression,4,7 whereas the heterotrimeric G-protein complex has been consistently shown to be a negative regulator of ABA signaling in these responses.6,10–13 ABA induces the dissociation of the GCR2-GPA1 complex, presumably the activation of the G-proteins, and consequently affects the reporter gene expression in yeasts.4 If GCR2 indeed functions as a GPCR for ABA and a positive regulator of ABA signaling as proposed,4 it is unclear how GCR2 functions through the heterotrimeric G-protein complex, a negative regulator of ABA signaling, to transduce ABA signal in these responses. The negative regulation of downstream signaling by the ligand-occupied receptor is not unprecedented. For example, such a mechanism is used in the ethylene signal transduction pathway.14 However, an inhibitory effect between a 7TM GPCR and the coupled heterotrimeric G-protein complex has not been documented. Taken together, GCR2 is not likely a 7TM GPCR but is more likely an Arabidopsis homolog of bacterial LanC.6,7
Arabidopsis GPA1 is an Unusual Heterotrimeric Gα Subunit
While the structure and functionality of GCR2 as a bona fide GPCR are still puzzling, Johnston et al., provided evidence that the heterotrimeric G-proteins likely act without a receptor guanine-nucleotide exchange factor (GEF), traditionally a GPCR in Arabidopsis.5 Johnston et al., used biochemical assays to examine the kinetics of GTP binding and GTP hydrolysis of recombinant GPA1 proteins. It was found that the binding of guanosine γ thio-phosphate (GTPγS), a non-hydrolyzable GTP analog, to GPA1 is over 20-fold faster than the most rapidly exchange Gα subunits previously described, namely human GαoA. On the other hand, GPA1 was shown to be among the slowest heterotrimeric GTPase described. Based on these biochemical parameters, it was calculated the rate of GTP hydrolysis by GPA1 is over two orders of magnitude slower than the rate of nucleotide exchange. Under steady state conditions, the percentage of GPA1 protein bound to GTP is predicted to be 99%, in contrast to that of <10% for any other Gαs described thus far. These results suggested that the GPA1 is in the activated state (GTP-bound) by default. Therefore, in striking contrast to that in metazoans, the rate-limiting step in the guanine nucleotide cycle of GPA1 is likely at the GTP hydrolysis, rather than GDP release. These unusual biochemical properties of GPA1 with high spontaneous nucleotide exchange coupled with slow GTP hydrolysis imply that unlike in the classical G-protein signaling paradigm, the GEF activity of GPCRs may not be required for the activation of G-protein signaling in Arabidopsis because GPA1 is already constitutively activated. The implication of this finding is that the heterotrimeric G-proteins likely act without any canonical GPCRs in Arabidopsis.
How does this finding fit in with our current knowledge of heterotrimeric G-protein signaling in Arabidopsis? The Arabidopsis genome encodes only one canonical Gα, one Gβ and two Gγ subunits.1,2 No GPCR has been unequivocally identified in plants, although several dozens of genes in the Arabidopsis genome encoding proteins with predicted topology of exactly 7TM regions and an extracellular amino-terminus.15 Among these 7TM proteins, AtRGS1 and GCR1 have been shown to physically interact with GPA1.11,16 However, no ligand has been identified and no GEF activity towards GPA1 has been demonstrated by either AtRGS1 or GCR1. Further, there are no Arabidopsis homologs of G-protein-coupled-receptor kinases (GRKs) or arrestins that specifically target GPCRs for desensitization in mammals.17 The unusual biochemical properties of GPA1 suggest that proteins possessing specific GTPase accelerating protein (GAP) activity towards GPA1 are more critical for the guanine nucleotide cycle of GPA1 and consequently the regulation of G-protein signaling in Arabidopsis. One specific class of such proteins in mammals are Regulator of G-protein Signaling (RGS) proteins.18
An Unexpected Heterotrimeric G-Protein-Signaling Mechanism in Arabidopsis
The Arabidopsis genome encodes a single RGS protein, AtRGS1. AtRGS1 is a unique RGS protein because it contains an N-terminal 7TM domain. The C-terminal RGS domain of AtRGS1 has been shown to exhibit GAP activity on GPA1,5,16 consistent with the known biology of RGS proteins. As discussed above, the unusual biochemical properties of GPA1 suggested that the rate-limiting step in the guanine nucleotide cycle of GPA1 is likely at the GTP hydrolysis. Therefore, one would expect that the GAP activity of AtRGS1, the sole RGS protein in Arabidopsis, towards GPA1 is critical for the regulation of G-protein signaling in Arabidopsis. To test this, a mutated form of AtRGS1, AtRGS1(E320K), that contains a point mutation in the RGS domain of AtRGS1 disrupting the Gα-binding interface and eliminating the GAP activity of AtRGS1, was used to examine its impact on G-protein-mediated hypocotyl growth and G-protein-mediated D-glucose sensing in Arabidopsis.5 As expected, overexpression of AtRGS1(E320K) failed to complement the Atrgs1 null alleles and was unable to confer D-glucose hypersensitivity that was typically observed in plants overexpressing wild-type form of AtRGS1, suggesting that the function of AtRGS1 in D-glucose sensing is dependent on its GAP activity on GPA1. Further, Atrgs1 gpa1 double mutant phenocopied gpa1 single mutant's D-glucose hypersensitivity phenotype, suggesting that the function of AtRGS1 in glucose sensing also depends on the presence of a functional GPA1. Taken together, these results support the hypothesis that the GAP activity is an essential regulatory function of AtRGS1 in the G-protein signal transduction in D-glucose sensing in Arabidopsis.
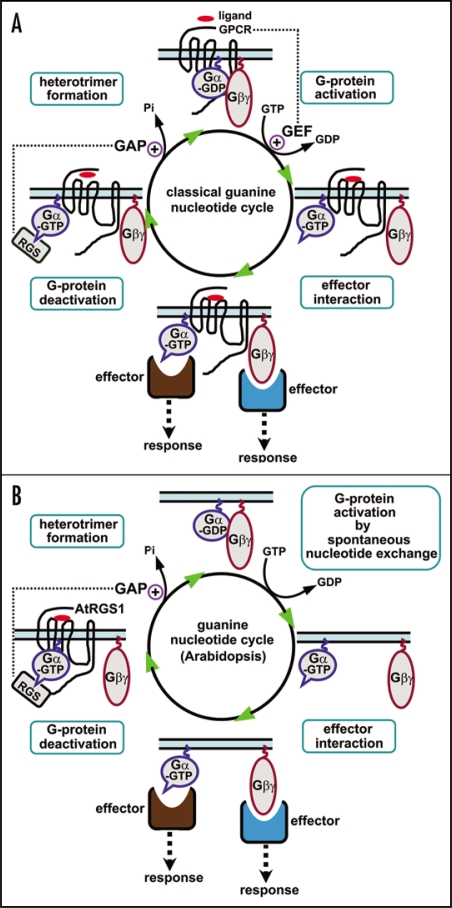
These findings provide an alternative mode of action of the heterotrimeric G-proteins. In a classical G-protein signaling paradigm, the activation of G-protein signaling relies on the GEF activity of GPCRs (controlled by ligand occupancy), and the RGS proteins (possessing GAP activity) deactivate the G-protein signaling by accelerating the intrinsic GTPase activity of Gα (Fig. 1A). In Arabidopsis, the G-protein signaling is constitutively activated due to a spontaneous nucleotide exchange and the slowest GTPase activity of Gα (GPA1). Therefore a classical GPCR with GEF activity may not be required for the activation of G-protein signaling in Arabidopsis. Instead, the G-protein signaling is critically regulated by the RGS protein, AtRGS1 (Fig. 1B).
Figure 1.
Modes of action of the heterotrimeric G-proteins. (a) A classical guanine nucleotide cycle of heterotrimeric Gα in animals. Heterotrimeric G-proteins, composed of Gα, Gβ and Gγ subunits, couple a specific class of cell-surface receptors, seven transmembrane (7TM) G-protein-coupled receptors (GPCRs). Upon ligand occupancy, GPCRs function as guanine-nucleotide exchange factors (GEFs) to promote the exchange of GDP for GTP on Gα, which subsequently causes the dissociation of Gβγ dimer from Gα. The activated Gα (GTP-bound) and liberated Gβγ dimer can then interact with downstream effectors, resulting in corresponding cellular responses. The GTP-bound Gα is returned to its inactive form (GDP-bound) by its intrinsic GTPase activity. The Regulator of G-protein Signaling (RGS) proteins possess GTPase accelerating protein (GAP) activity on Gα. (b) Guanine nucleotide cycle of heterotrimeric Gα in Arabidopsis. Arabidopsis genome encodes one canonical Gα (GPA1), one Gβ (AGB1) and two Gγ (AGG1 and AGG2) subunits. GPA1 is an unusual heterotrimeric Gα because of a rapid nucleotide exchange and the slowest GTPase activity. Therefore, the GTP hydrolysis, rather than GDP release, is likely the rate-limiting step in the guanine nucleotide cycle of GPA1. Classical GPCRs (possessing GEF activity on Gα) may not be required for the activation of G-protein signaling because GPA1 is in the activated state (GTP-bound) by default. Instead, AtRGS1, the sole RGS protein in Arabidopsis, functions as a critical regulator of G-protein signaling by accelerating the slow, intrinsic GTPase activity of GPA1. It is likely that AtRGS1 acts as a ligand-regulated 7TM GAP for GPA1. The exact ligand for AtRGS1 that modulates GAP activity has not been identified, though D-glucose has been shown to stimulate the interaction between AtRGS1 and GPA1. Upon activation, Gα and Gβγ subunits are shown to dissociate in this model, but Arabidopsis G-proteins may signal through nondissociable heterotrimers.21
Future Perspective
Although the repertoire of the heterotrimeric G-protein complex appears to be much simpler in plants compared with that in mammals, our knowledge about the heterotrimeric G-protein signaling mechanism in plants is still very limited. There is urgent need to identify new components of the heterotrimeric G-protein signaling complex in plants. The unique and unusual biochemical properties of the sole Arabidopsis heterotrimeric Gα challenge our fundamental understanding of the heterotrimeric G-protein signaling paradigm developed in mammals. However, the unexpected model of heterotrimeric G-protein signaling and its predictions need to be tested further in planta. It is unknown if such an unexpected signaling mechanism in Arabidopsis represents a general mode of action of the heterotrimeric G-proteins in plants. This finding also raises the question of how to evaluate the role of 7TM proteins in G-protein signaling. A known G-protein-coupled 7TM protein in Arabidopsis, GCR1, has been shown to act in both G-protein-dependent and G protein-independent pathways.13,19 The unusual and unexpected biochemical properties of GPA1 may explain the difficulty for identifying a bona fide classical GPCR in Arabidopsis, and raising the possibility that while 7TM receptors can act at ‘zero G’,20 G-proteins can act at ‘zero GPCR’ as well.
Acknowledgements
I thank Dr. Francis Willard and Dr. Alan Jones (University of North Carolina at Chapel Hill) for invaluable comments. Work in my laboratory on heterotrimeric G-proteins is supported by grants from NSERC and CFI.
Abbreviations
- 7TM
seven transmembrane
- G-proteins
heterotrimeric G-proteins
- GPCR
G-protein-coupled receptor
- ABA
abscisic acid
- Gα
heterotrimeric G-protein α subunit
- LanC
lanthionine synthetase
- GAP
GTPase accelerating protein
- GEF
guanine-nucleotide exchange factor
- RGS
regulator of G-protein signaling
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6064
References
- 1.Assmann SM. Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell. 2002;14:355–373. doi: 10.1105/tpc.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temple BRS, Jones AM. The plant heterotrimeric G-protein complex. Annu Rev Plant Biol. 2007;58:249–266. doi: 10.1146/annurev.arplant.58.032806.103827. [DOI] [PubMed] [Google Scholar]
- 3.Perfus-Barbeoch L, Jones AM, Assmann SM. Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol. 2004;7:719–731. doi: 10.1016/j.pbi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 5.Johnston CA, Taylor JP, Gao Y, Kimple AJ, Grigston JC, Chen JG, Siderovski DP, Jones AM, Willard FS. GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc Natl Acad Sci USA. 2007;104:17317–17322. doi: 10.1073/pnas.0704751104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston CA, Temple BR, Chen JG, Gao Y, Moriyama EN, Jones AM, Siderovski DP, Willard FS. Comment on ‘A G Protein-Coupled Receptor Is a Plasma Membrane Receptor for the Plant Hormone Abscisic Acid’. Science. 2007;318:914. doi: 10.1126/science.1143230. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Zeng Q, Guo J, Cheng J, Ellis BE, Chen JG. Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. Plant J. 2007;52:1001–1013. doi: 10.1111/j.1365-313X.2007.03291.x. [DOI] [PubMed] [Google Scholar]
- 8.Illingworth CJ, Parkes KE, Snell CR, Mullineaux PM, Reynolds CA. Criteria for confirming sequence periodicity identified by Fourier transform analysis: Application to GCR2, a candidate plant GPCR? Biophys Chem. 2008;133:28–35. doi: 10.1016/j.bpc.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Yu JP, Brunzelle JS, Moll GN, van der Donk WA, Nair SK. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science. 2006;311:1464–1467. doi: 10.1126/science.1121422. [DOI] [PubMed] [Google Scholar]
- 10.Ullah H, Chen JG, Wang S, Jones AM. Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol. 2002;129:897–907. doi: 10.1104/pp.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey S, Assmann SM. The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell. 2004;16:1616–1632. doi: 10.1105/tpc.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assmann SM. G protein signaling in the regulation of Arabidopsis seed germination. Sci STKE. 2005 doi: 10.1126/stke.3082005cm11. cm11. [DOI] [PubMed] [Google Scholar]
- 13.Pandey S, Chen JG, Jones AM, Assmann SM. G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol. 2006;141:243–256. doi: 10.1104/pp.106.079038. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chow B, McCourt P. Plant hormone receptors: perception is everything. Genes Dev. 2006;20:1998–2008. doi: 10.1101/gad.1432806. [DOI] [PubMed] [Google Scholar]
- 15.Moriyama EN, Strope PK, Opiyo SO, Chen Z, Jones AM. Mining the Arabidopsis thaliana genome for highly-divergent seven transmembrane receptors. Genome Biol. 2006;7:96. doi: 10.1186/gb-2006-7-10-r96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JG, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovski DP. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science. 2003;301:1728–1731. doi: 10.1126/science.1087790. [DOI] [PubMed] [Google Scholar]
- 17.Moore CAC, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Ann Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 18.Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- 19.Chen JG, Pandey S, Huang J, Alonso JM, Ecker JR, Assmann SM, Jones AM. GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol. 2004;135:907–915. doi: 10.1104/pp.104.038992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brzostowski JA, Kimmel AR. Signaling at zero G: G-protein-independent functions for 7-TM receptors. Trends Biochem Sci. 2001;26:291–297. doi: 10.1016/s0968-0004(01)01804-7. [DOI] [PubMed] [Google Scholar]
- 21.Adjobo Hermans MJ, Goedhart J, Gadella TW., Jr Plant G protein heterotrimers require dual lipidation motifs of Gα and Gγ and do not dissociate upon activation. J Cell Sci. 2006;119:5087–5097. doi: 10.1242/jcs.03284. [DOI] [PubMed] [Google Scholar]