Abstract
Aquaporins, which facilitate the diffusion of water across biological membranes, are key molecules for the regulation of water transport at the cell and organ levels. We recently reported that hydrogen peroxide (H2O2) acts as an intermediate in the regulation of Arabidopsis root water transport and aquaporins in response to NaCl and salicylic acid (SA).1 Its action involves signaling pathways and an internalization of aquaporins from the cell surface. The present addendum connects these findings to another recent work which describes multiple phosphorylations in the C-terminus of aquaporins expressed in the Arabidopsis root plasma membrane.2 A novel role for phosphorylation in the process of salt-induced relocalization of AtPIP2;1, one of the most abundant root aquaporins, was unraveled. Altogether, the data delineate reactive oxygen species (ROS)-dependent signaling mechanisms which, in response to a variety of abiotic and biotic stresses, can trigger phosphorylation-dependent PIP aquaporin intracellular trafficking and root water transport downregulation.
Key words: reactive oxygen species, aquaporin, phosphorylation, cell signaling, stress, protein relocalization, root water transport
Plants can regulate their water uptake capacity i.e. their root hydraulic conductivity (Lpr) on a short term (minutes to hour) basis through regulation of plasma membrane (PM) aquaporins of the Plasma membrane Intrinsic Protein (PIP) subfamily.3 It has been known for a long time that salt stress (NaCl), as many other abiotic stresses such as cold, anoxia or nutrient deprivation, induces an inhibition of Lpr in many plant species.3 In the recent study by Boursiac et al. (2008),1 we identified SA as a new inhibitory increased the accumulation of ROS in roots, it was hypothesized that H2O2 or other ROS may have a central role in the regulation of root water transport in response to various biotic or abiotic stimuli. When Arabidopsis roots were treated with mM concentrations of exogenous H2O2, Lpr was inhibited within minutes by up to 90%. These findings are consistent with previous reports showing that ROS can downregulate water transport in cucumber and maize roots or in the algae Chara corallina.4–7 H2O2 and possibly other derived ROS may modulate the Lpr through signaling mechanisms or by a direct oxidative gating of aquaporins. The latter hypothesis, which has been favored in previous studies by Steudle and colleagues,6,7 was investigated by Boursiac et al., by functionally expressing aquaporins in Xenopus oocytes and by testing their sensitivity to external H2O2. The results show that Arabidopsis aquaporins are insensitive to direct oxidation by H2O2 or hydroxyl radicals. Thus, these and complementary pharmacological analyses on excised roots rather support a role for H2O2 as a second messenger that connects environmental stimulus perception to water transport regulation in plant roots. The additional finding that H2O2 can be transported by aquaporins8,9 opens the possibility of intricate loop mechanisms whereby these proteins may interfere with their own regulation. For example, active PIP aquaporins could facilitate the diffusion within the cell of NADPH-oxidase derived apoplastic H2O2, which in turn would activate signaling pathways acting on PIP activity and/or subcellular localization.
In a previous study, we monitored the subcellular localization of AtPIP1;2 and AtPIP2;1, two of the most abundant PIPs in roots, by expression in transgenic Arabidopsis of fusions with the green fluorescent protein (GFP).10 We observed that a 100 mM NaCl treatment induced in 2–4 hours an increased intracellular labeling which was interpreted as an intracellular relocalization of the two aquaporins.10 In our more recent study, both a 150 mM NaCl and a 0.5 mM SA treatments induced an intracellular labeling by GFP-PIP1;2 and PIP2;1-GFP fusions, with a “fuzzy” pattern or at the level of spherical bodies. Preventing the NaCl- or SA-dependent accumulation of ROS with exogenous catalase was able to almost completely counteract the effects of the two stimuli on the localization pattern of the PIP2;1-GFP fusion. In addition, the inhibition of Lpr by SA was also counteracted at 33% by the catalase treatment. Altogether, the data stress the importance of an ROS-induced relocalization of aquaporins in the regulation of root water transport. Yet, we still miss quantitative data and complementary pharmacological evidence to determine the exact contribution of aquaporin relocalization with respect to other aquaporin regulatory mechanisms.
Another recent work by our group has, however, provided deeper insights into the mechanisms of stress-induced relocalization of aquaporins in plants.2 Our group identified by mass spectrometry multiple adjacent phosphorylation sites (up to 4 in the case of AtPIP2;4) in the C-terminus of aquaporins expressed at the root plasma membrane.2 Phosphorylation of AtPIP2;1, which shows a simpler profile with only two sites at Ser280 and Ser283, was studied in closer detail by site-directed mutagenesis and expression in transgenic Arabidopsis of GFP-PIP2;1 fusions. A Ser283Ala mutation, which mimics a constitutively dephosphorylated Ser283, induced a marked intracellular accumulation of GFP-PIP2;1 in resting conditions. Because no phenotype was observed after a Ser280Ala mutation, the data suggest a specific role for Ser283 phosphorylation in the proper targeting of the protein. When plants were treated by 100 mM NaCl for 2 to 4 hours, the wild type (WT) and Ser280Ala mutant forms of GFP-PIP2;1 showed similar intracellular staining, in both “fuzzy” structures or spherical bodies. On the contrary, the Ser283Ala mutant did not label any spherical body. Interestingly, a Ser283Asp mutation that mimics a constitutively phosphorylated Ser283 resulted in a salt-induced labeling of spherical bodies similar to the one observed with WT GFP-PIP2;1 whereas no “fuzzy” staining was observed. Therefore, the phosphorylation status of Ser283 seems to determine the redistribution of AtPIP2;1 towards fuzzy structures (non-phosphorylated Ser283) or spherical bodies (phosphorylated Ser283). Although the nature of these intracellular structures remains to be identified, we now consider the possibility that the spherical bodies correspond to the late endosome/prevacuolar compartment that orientates aquaporins towards a degradation pathway whereas the fuzzy structures may act as a storage compartment for subsequent relocalization of PIP aquaporins to the PM, and rapid recovery of the PM water permeability. Although we favor the idea that the intracellular labeling shown by GFP-PIP2;1 in response to salt originates from aquaporins relocalized from the PM, newly synthesized proteins may also contribute to this pattern.
Prak et al., also developed an absolute quantification method to show that the phosphorylation profile of AtPIP2;1 at the root plasma membrane was altered upon 100 mM NaCl and 2 mM H2O2 treatments. Whereas NaCl decreased the abundance of phosphorylated Ser283, H2O2 enhanced the overall phosphorylation of the AtPIP2;1 C-terminus. These observations add another level of complexity to the mechanisms of stimulus-induced and phosphorylation- dependent relocalisation of plant aquaporins uncovered in our group. Although one of the primary effects of NaCl is undoubtedly an accumulation of ROS, the difference in phosphorylation patterns observed in response to H2O2 and NaCl treatments may come from quantitative and kinetic differences in ROS patterns between the two treatments or from additional regulations activated by salt.
We note that phosphorylation of PIP aquaporins had already been investigated in detail.11–13 In particular, studies with spinach SoPIP2;1 has pointed to two phosphorylation sites, Ser115 in the first cytoplasmic loop (loop B) and Ser274 at the C-terminus, as important for modulating the water transport activity of this aquaporin after expression in Xenopus oocytes. A role for these two sites in aquaporin gating was also deduced from the atomic structure of SoPIP2;1.14 Whereas Ser280 in AtPIP2;1 corresponds to Ser274 in SoPIP2;1, the functional role of sites equivalent to Ser283 in AtPIP2;1 had not been considered previously in any other PIP. To our knowledge, the study by Prak et al., provides the first evidence in plants for a role of phosphorylation on the relocalization of aquaporins and highlights the importance of multiple phosphorylations sites in the C-terminus of aquaporins, as has been recently shown in human Aquaporin-2.15,16
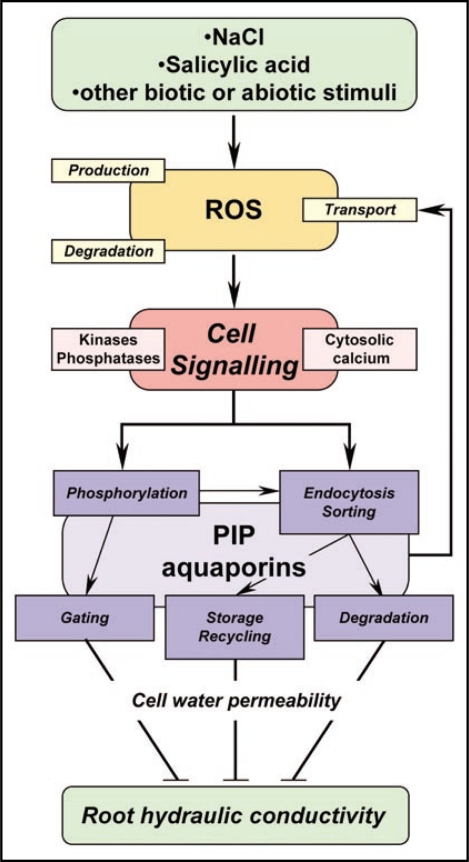
Overall, the advance provided by our two recent studies delineates a working model (Fig. 1), whereby multiple abiotic and biotic stresses, which all induce an accumulation of ROS, activate common signaling pathways to downregulate root water transport. We have provided evidence that some of these pathways are calcium- and/ or protein kinase-dependent. One regulatory mechanism triggered by these pathways is the relocalization of aquaporins into intracellular “fuzzy” structures or bigger spherical bodies. For AtPIP2;1, the sorting between these structures is determined in part by the phosphorylation status of Ser283, which ultimately may control the cellular fate of the protein for degradation or remobilization to the PM. A coming challenge will be to determine how this and other cellular mechanisms quantitatively contribute to the integrated regulation of water transport at the cell and tissue (whole root) levels. Another avenue for future research will be to identify the molecular components involved in upstream ROS-dependent cell signaling and aquaporin phosphorylation. These studies will tell us how the regulation of root water uptake in parallel to the regulation of transpiration allows the plant to preserve its water status when it is continuously challenged by multiple stresses.
Figure 1.
Tentative model of regulation of root hydraulic conductivity (Lpr) through reactive oxygen species (ROS) signaling. Multiple biotic and abiotic stimuli such as NaCl or salicylic acid can induce an intra- and/or extracellular accumulation of ROS by acting on their production, degradation or transport. The stimulus-induced ROS in turn activate signaling pathways involving protein kinases and cytosolic calcium. These events result in changes in the phosphorylation and subcellular localization patterns of plasma membrane (PM) aquaporins (PIPs). In particular, endocytosis can direct PIPs towards various intracellular compartments for subsequent recycling at the PM or degradation. Phosphorylation can interfere with this routing process, but also determines the intrinsic water transport activity (gating) of PM localized PIPs. The possibility exists that signaling components directly act on PIP gating, recycling or degradation through phosphorylation- and endocytosis-independent pathways (not shown). In addition, transport of H2O2 by PIP aquaporins may provide retroactive effects of aquaporins on upstream signaling events. Aquaporin activity at the PM determines root cell water permeability, which contributes to most of Lpr in Arabidopsis. The overall scheme shows how stress-induced ROS signaling results in an inhibition of PIP aquaporin activity and, as a consequence, in an overall downregulation of Lpr.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7002
References
- 1.Boursiac Y, Boudet J, Postaire O, Luu DT, Tournaire-Roux C, Maurel C. Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J. 2008;56:207–218. doi: 10.1111/j.1365-313X.2008.03594.x. [DOI] [PubMed] [Google Scholar]
- 2.Prak S, Hem S, Boudet J, Viennois G, Sommerer N, Rossignol M, Maurel C, Santoni V. Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: role in subcellular trafficking of AtPIP2;1 in response to salt stress. Mol Cell Proteomics. 2008;7:1019–1030. doi: 10.1074/mcp.M700566-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 4.Lee SH, Singh AP, Chung GC. Rapid accumulation of hydrogen peroxide in cucumber roots due to exposure to low temperature appears to mediate decreases in water transport. J Exp Bot. 2004;55:1733–1741. doi: 10.1093/jxb/erh189. [DOI] [PubMed] [Google Scholar]
- 5.Aroca R, Amodeo G, Fernandez-Illescas S, Herman EM, Chaumont F, Chrispeels MJ. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005;137:341–353. doi: 10.1104/pp.104.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Q, Steudle E. Oxidative gating of water channels (aquaporins) in corn roots. Plant Cell Environ. 2006;29:459–470. doi: 10.1111/j.1365-3040.2005.01423.x. [DOI] [PubMed] [Google Scholar]
- 7.Henzler T, Ye Q, Steudle E. Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant Cell Environ. 2004;27:1184–1195. [Google Scholar]
- 8.Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J. 2008;414:53–61. doi: 10.1042/BJ20080287. [DOI] [PubMed] [Google Scholar]
- 9.Henzler T, Steudle E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina. Model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot. 2000;51:2053–2066. doi: 10.1093/jexbot/51.353.2053. [DOI] [PubMed] [Google Scholar]
- 10.Boursiac Y, Chen S, Luu DT, Sorieul M, van den Dries N, Maurel C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005;139:790–805. doi: 10.1104/pp.105.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjovall-Larsen S, Alexandersson E, Johansson I, Karlsson M, Johanson U, Kjellbom P. Purification and characterization of two protein kinases acting on the aquaporin SoPIP2;1. Biochim Biophys Acta. 2006;1758:1157–1164. doi: 10.1016/j.bbamem.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Johansson I, Larsson C, Ek B, Kjellbom P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell. 1996;8:1181–1191. doi: 10.1105/tpc.8.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell. 1998;10:451–459. doi: 10.1105/tpc.10.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P. Structural mechanism of plant aquaporin gating. Nature. 2006;439:688–694. doi: 10.1038/nature04316. [DOI] [PubMed] [Google Scholar]
- 15.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pistikun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at ser-269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem. 2008;283:24617–24627. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA. 2008;105:3134–3139. doi: 10.1073/pnas.0712338105. [DOI] [PMC free article] [PubMed] [Google Scholar]