Abstract
Membranes are the primary sites of perception for extracellular stimuli and are rich sources for signaling messengers. Phospholipase D (PLD) hydrolyzes membrane lipids to produce the messenger phosphatidic acid (PA), and the activation of PLD occurs under different hyperosmotic stresses, including dehydration and salt stress. We have recently found that PLDα3 that plays a positive role in hyperosmotic stress. PLDα3 hydrolyzes multiple substrates with distinguishable preferences. The involvement of PLDα3 in hyperosmotic stress is through a different mechanism from that PLDα1, which mediates the effect of abscisic acid on stomatal movements. PLDα3 enhances root growth and accelerates flowering time under hyperosmotic stress. Alterations of PLDα3 affect the level of PA, transcripts of TOR and AGC2.1, ABA-responsive genes, and phosphorylated S6K protein under hyperosmotic stress. Our further observation shows that PLDα3 is also involved in glucose response. PLDα3-KO seeds and seedlings are less sensitive to glucose whereas PLDα3-overepressed seeds are more sensitive than wild type. These results point to a possibility that PLDα3-mediated lipid signaling may play a role in integrating nutrient sensing, protein kinase activation, and hormones responses to regulate growth and development under hyperosmotic stress.
Key words: hyperosmotic stress, phosphatidic acid, TOR, S6K, lipid signaling, phospholipase D, glucose sensing
Hyperosmotic stress is a critical factor that limits plant growth and agricultural productivity. Plants experience hyperosmotic stress under different growth conditions including high salinity and drought. Plants have evolved to adapt various stress environments through changes in morphological, physiological, biochemical or molecular response.1,2 Several classes of regulatory components, such as plant hormones, transcription factors, proteins kinases, and Ca2+ play important roles in plant response to salinity or drought signaling processes.3–5 Increasing results show that membrane lipids are rich sources for signaling messengers.6–8 Phospholipase D (PLD) hydrolyzes membrane phospholipids to generate phosphatidic acids (PA), a signaling molecule involved in a variety of biological processes, such as freezing,9 auxin and vesicular trafficking,10 root hair growth,11,12 ABA signaling in stomatal movement,13,14 and phosphorus starvation.15,16 The activation of PLD and PA elevation occur in plants under hyperosmotic stress such as dehydration17 and salt treatment.18,19 However, the physiological effect of the PLD activation and the role of specific PLDs in responses to salinity and water deficit are largely unknown.
Plant PLD consists of a family of heterogenous enzymes. Arabidopsis has 12 PLDs, including 10 C2-PLDs with α (3), β (2), γ (3), δ and ε and two PH/PX-PLDζ1 and PLDζ2.20 PLDα1 is the most abundant PLD in plants and is involved in plant water loss. PLDα1 plays an important role in stomatal movements through mediating ABA signaling.13,14 PLDα1-derived PA tethers ABI1 to membrane to sequester the negative effect of ABI1 on ABA stimulated stomatal closure.13 Of the three PLDs in the α group, PLDα3 is more distantly related to PLDα1 than is PLDα2. We have recently found that PLDα3 plays a positive role in hyperosmotic stress.22 PLDα3-knockout (KO) plants are less tolerant to salt stress than WT plants. In addition, under water deficit conditions, PLDα3-KO plants flower later, whereas PLDα3-overexpressed (OE) plants flower earlier than WT plants. Unlike PLDα1 that is involved in stomatal movement through mediating ABA signaling,13,14 alteration of PLDα3 does not change stomatal movement and water loss,22 suggesting that PLDα3 is involved in hyperosmotic stress response in a mechanism different from that of PLDα1. PLDα3-KO plants are capable of ABA accumulation induced by hyperosmotic stress. But PLDα3-KO plants display higher levels of ABA-responsive gene expression and ABA inhibitions on seedling growth than WT plants. PLDα3-KO plants have fewer and shorter roots, whereas OE plants have more and longer roots than WT plants under hyperosmotic stress. Collectively, these results suggest that PLDα3 promotes root growth to enhance hyperosmotic tolerance.22
Biochemical analysis shows that PLDα3 uses multiple substrates with distinguishable preferences.22 Results of lipid profiling indicate that PLDα3-KO plants accumulate less PA, suggesting that PLDα3 contributes to PA formation under hyperosmoitc stress.22 PA has been found to be an activator of several Ser/Thr protein kinases involved in organismal growth. In plants, PA activates PDK1 to phosphorylate AGC2.1 and promotes root hair growth.12 In animals, PLD1-derived PA activates mammalian target of rapamycin (mTOR) kinase to phosphorylate downstream kinase, ribosomal S6 kinase (S6K), PA can also directly interact with and activate S6K to enhance cell growth.23,24 However, the linkage between PA and TOR-S6K pathway in plants remains unknown. Further analysis shows that KO of PLDα3 renders plants lower, whereas OE plants have higher levels of phosphorylated S6K protein and transcripts of TOR and AGC 2.1 than WT under hyperosmotic stress.22 These results raise an intriguing question of whether PLDα3 is involved in the activation of Ser/Thr protein kinases, thus regulating plants growth and development under hyperosmotic stress.
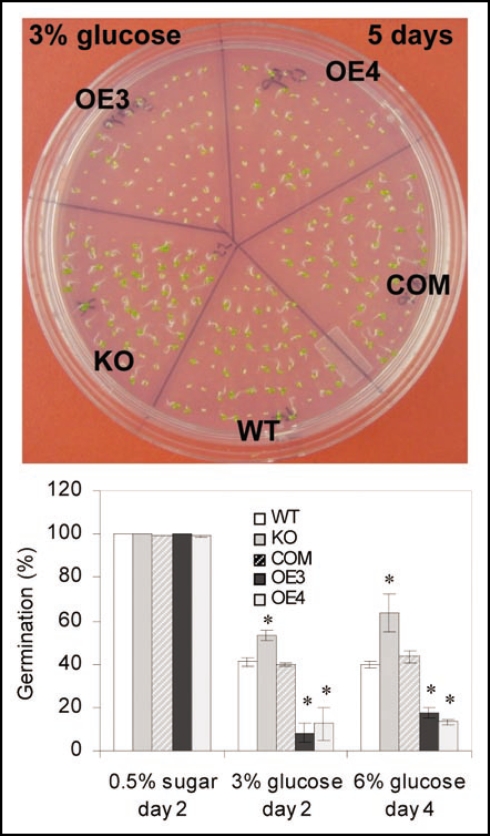
In addition, our recent results show that alterations of PLDα3 result in changes in glucose sensitivity (Fig. 1). When seeds are germinated in MS containing 3 and 6% glucose, PLDα3-KO seeds and seedlings are less sensitive to glucose, as indicated by the earlier germination and less glucose inhibition of growth, whereas OE of PLDα3 enhances glucose sensitivity, as indicated by delayed germination and greater inhibition of seedling growth and development (Fig. 1). The effect of glucose on seed germination and seedling growth is not due to hyperosmotic stress imposed by glucose because the effect is opposite to that under hyperomotic stress.22 Glucose is not only a metabolite, but also is an important signaling molecule involved in growth, development and stress response.25 An Arabidopsis defect in glucose sensing causes plant growth retardation.25 PLDα3 may be involved in the crosstalk among glucose sensing, ABA response, and S6K activation to regulate growth and development. It will be of interest in future studies to investigate the complex network between lipid signaling, Ser/Thr protein kinase, and nutrient sensing and hormone response in plants.
Figure 1.
Changes in glucose sensitivity in PLDα3-KO and OE seedlings. Seeds were germinated in MS containing 3% and 6% glucose. Values are means ± SD (n = 3) of three experiments. Each genotype contained at least 100 seeds in each experiment.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7003
References
- 1.Vij S, Tyagi AK. Emerging trends in the functional genomics of the abiotic stress response in crop plants. Plant Biotechnol J. 2007;5:361–380. doi: 10.1111/j.1467-7652.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 2.Sreenivasulu N, Sopory SK, Kavi Kishor PB. Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene. 2007;388:1–13. doi: 10.1016/j.gene.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Jonak C, Okresz L, Bogre L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol. 2002;5:415–424. doi: 10.1016/s1369-5266(02)00285-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J. Salt and drought stress signal transduction in plants. Annu Rev Plant Boil. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Wang X. Lipid signaling. Curr Opin Plant Biol. 2004;7:1–8. doi: 10.1016/j.pbi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Testerink C, Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005;10:368–375. doi: 10.1016/j.tplants.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Li M, Zhang W, Wang X. The plasma membrane-bound phospholipase Dδ enhances freezing tolerance in Arabidopsis thaliana. Nat Biotechnol. 2004;22:427–433. doi: 10.1038/nbt949. [DOI] [PubMed] [Google Scholar]
- 10.Li G, Xue HW. Arabidopsis PLDζ2 regulates vesicle trafficking and is required for auxin response. Plant Cell. 2007;19:281–295. doi: 10.1105/tpc.106.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, Rubert I, Morelli G, Aoyama T. Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science. 2003;300:1427–1430. doi: 10.1126/science.1083695. [DOI] [PubMed] [Google Scholar]
- 12.Anthony RG, Henrigues R, Helfer A, Meszaros T, Rios G, Testerink G, Munnik T, Deak M, Koncz C, Bogre L. A protein kinase target of a PDK1 signalling pathway is involved in root hair growth in Arabidopsis. EMBO J. 2004;23:572–581. doi: 10.1038/sj.emboj.7600068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Qin C, Zhao J, Wang X. Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA. 2004;101:9508–9513. doi: 10.1073/pnas.0402112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra G, Zhang W, Deng F, Zhao J, Wang X. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science. 2006;312:264–266. doi: 10.1126/science.1123769. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Qin C, Welti R, Wang X. Double knockouts of phospholipases Dζ1 and Dζ2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiol. 2006;140:761–770. doi: 10.1104/pp.105.070995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Ramirez A, Oropeza-Aburto A, Razo-Hernandez F, Ramirez-Chavez E, Herrera-Estrella L. Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Arabidopsis roots. Proc Natl Acad Sci USA. 2006;103:6765–6770. doi: 10.1073/pnas.0600863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katagiri T, Takahashi S, Shinozaki K. Involvement of a novel Arabidopsis phospholipase D, At PLDδ, in dehydration-inducible accumulation of phosphatidic acid in stress signaling. Plant J. 2001;26:595–605. doi: 10.1046/j.1365-313x.2001.01060.x. [DOI] [PubMed] [Google Scholar]
- 18.Frank W, Munnik T, Kerkmann K, Salamini F, Bartel D. Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell. 2000;12:111–124. doi: 10.1105/tpc.12.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munnik T, Meijer H, Riet BT, Hirt H, Frank W, Bartels D, Musgrave A. Hyperosmotic stress stimulates phospholipase D activity and elevates the level of phosphatidic acid and diacylglycerol pyrophosphate. Plant J. 2000;22:147–154. doi: 10.1046/j.1365-313x.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- 20.Qin C, Wang X. The Arabidopsis phospholipase D family. Characterization of a calcium-independent and phosphatidylcholine-selective PLDζ1 with distinct regulatory domains. Plant Physiol. 2002;128:1057–1068. doi: 10.1104/pp.010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G, Lin F, Xue HW. Genome-wide analysis of the phospholipase D family in Oryza sativa and functional characterization of PLD beta1 in seed germination. Cell Res. 2007;17:881–894. doi: 10.1038/cr.2007.77. [DOI] [PubMed] [Google Scholar]
- 22.Hong Y, Pan X, Welti R, Wang X. Phospholipase Dα3 is involved in the hyperosmotic response in Arabidopsis. Plant Cell. 2008;20:803–816. doi: 10.1105/tpc.107.056390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y, Vilella-Bach M, Barchmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 24.Lehman N, Ledford B, Di Fulvio M, Frondorf K, McPhail LC, Gomez-Cambronero J. Phospholipase D2-derived phosphatidic acid binds to and activates ribosomal p70 S6 kinase independently of mTOR. FASEB J. 2007;21:1075–1087. doi: 10.1096/fj.06-6652com. [DOI] [PubMed] [Google Scholar]
- 25.Cho YH, Yoo SD, Sheen J. Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell. 2006;127:579–589. doi: 10.1016/j.cell.2006.09.028. [DOI] [PubMed] [Google Scholar]