Abstract
In our recent paper in the Journal of Experimental Botany, we demonstrated that Brassica juncea BjCHI1 shows anti-fungal properties against phytopathogens, Colletotrichum truncatum, C. acutatum, Botrytis cinerea and Ascochyta rabiei. Furthermore, BjCHI1 which is an unusual plant chitinase with two (almost identical) chitin-binding domains, agglutinates Gram-negative bacteria, adversely affecting their growth. In contrast, BjCHI1 derivatives lacking one or both domains do not show agglutination activity, suggesting that both chitin-binding domains are essential for agglutination. Observations that agglutination could be relieved by addition of galactose, glucose or lactose, imply that BjCHI1 interacts with the carbohydrate components of the Gram-negative bacterial cell wall. We propose here, a model for BjCHI1-mediated agglutination between Gram-negative bacteria, through interaction of their adjacent cell walls mediated by the two chitin-binding domains of BjCHI1. BjCHI1 is a plant chitinase which has evolved towards acquiring an enhanced role in plant defense against fungi and Gram-negative bacteria. Hence, it is a promising candidate for applications against phytopathogens in plant genetic engineering via nuclear or plastid transformation.
Key words: bacterial agglutination, chitin-binding domain, Indian mustard, lectin, phytopathogens, Pichia-expressed proteins, transplastomic tobacco
An Unusual Chimera Consisting of Two Chitin-Binding Domains Linked to a Chitinase Catalytic Domain
BjCHI1, the first chitinase identified to possess two chitin-binding domains, is an unusual chimera that also includes a chitinase catalytic domain.1 It can be classified as a plant lectin because lectins are defined to contain one or more chitin-binding domains.2,3 BjCHI1 also displays chitinase activity by virtue of a chitinase catalytic domain residing at its C-terminus.1,4–6 Structurally, it differs from Class I chitinases7 which contain only one chitin-binding domain, and from other plant lectins, such as hevein8 which has a single chitin-binding domain, and wheat germ agglutinin which has four such domains.9 The two latter proteins lack the catalytic domain and thus do not show chitinase activity.
BjCHI1 is a New Member of Hololectins that can Agglutinate Bacteria
Our investigations using both Pichia pastoris-expressed recombinant BjCHI1 and plant extracts from transplastomic tobacco overexpressing BjCHI1 indicate that BjCHI1 agglutination activity is correlated with growth inhibition of Gram-negative bacteria. BjCHI1 inhibited the growth of Gram-negative bacteria (Escherichia coli, Ralstonia solanacearum, Pseudomonas aeruginosa) more effectively than Gram-positive bacteria (Micrococcus luteus and Bacillus megaterium). Notably, this study demonstrates the significance of the second chitin-binding domain in conferring agglutination. BjCHI derivatives lacking one or both chitin-binding domains did not display bacterial inhibition in all strains of bacteria tested. These results are consistent with our previous observations on the agglutination of rabbit erythrocytes, evident only in BjCHI1, but not in its derivatives lacking one or both chitin-binding domains.6 They are also in good agreement with published data on the agglutination function of other carbohydrate-binding proteins.3,9,10 Plant lectins are defined as “plant proteins possessing at least one non-catalytic domain, which binds reversibly to a specific mono-or oligosaccharide”.10 However, a monovalent lectin with merely a single carbohydrate-binding domain (i.e., merolectin) does not precipitate glycoconjugates or agglutinate cells.
Previous studies have shown that the subgroup, hololectin, may effectively agglutinate cells.11 Hololectins contain two or more carbohydrate-binding domains which are identical or very homologous. Their multiple binding sites allow them to fully agglutinate cells. Interestingly, the amino acid sequence of the two chitin-binding domains of BjCHI1 shows 95% identity with each other, which indicates homologous binding specificity,1 suggestive that BjCHI1 is a new member of the hololectins. The bivalent structure of BjCHI1 may be a prototype of plant pathogenesis-related proteins that are able to agglutinate and inhibit bacteria cell growth. The precursor of Urtica dioica agglutinin (UDA), which resembles BjCHI1 in having two chitin-binding domains,1 has already been grouped to hololectins.11 However, UDA differs from BjCHI1 because its catalytic domain is eliminated from the two chitin-binding domains during posttranslational cleavage.
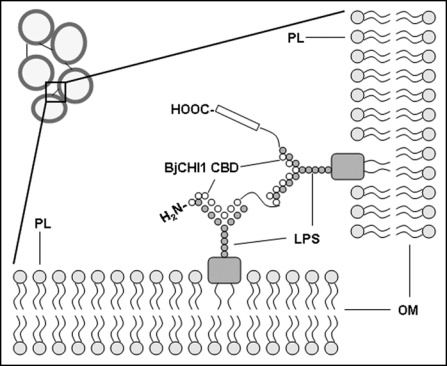
Inhibition of agglutination by free monosaccharides and oligosaccharides suggests of interactions between the BjCHI1 lectin and lipopolysacharide moieties residing on Gram-negative bacterial cell walls. We propose a model for this BjCHI1-mediated agglutination involving the outer membranes of adjacent cell walls of Gram-negative bacteria (Fig. 1). Such agglutination would be effective in immobilizing motile bacteria. Agglutination of R. solanacearum was relieved by the addition of carbohydrates: free monosaccharides (galactose and glucose) and disaccharide (lactose), implying presence of lectinlipopolysaccharide interactions between BjCHI1 and Gram-negative bacterial cell walls. The outer core of lipopolysaccharides on the bacterial cell wall consists of pentasaccharide glucose, galactose and N-acetylglucosamine, and the O-antigen includes mannose and rhamnose; most interestingly, chitin-binding lectins containing hevein domains are N-acetylglucosamine-specific.12
Figure 1.
Model showing how BjCHI1 can mediate agglutination of Gram-negative bacteria. The two near identical chitin-binding domains (CBD) bind carbohydrates and are responsible for linking lipopolysaccharide (LPS) on the outer membranes (OM) of the cell walls of adjacent Gram-negative bacteria. An agglutinated matrix of bacteria results from this BjCHI1-mediated action which can be relieved by the addition of galactose, glucose or lactose. PL, phospholipid.
Chitinase Activity is Independent on the Number of Chitin-Binding Domains
In the current study, the derivative of BjCHI1 lacking a second chitin-binding domain, also showed growth inhibition of fungal hyphae of C. truncatum and C. acutatum. This is in accordance with previous studies demonstrating that this derivative, when expressed and extracted from transgenic tobacco, showed levels of chitinase activity similar to transgenic tobacco-derived BjCHI1 in standard colorimetric chitinase assays.4 Multiple chitin-binding domains do not seem to be required for antifungal chitinase activity. Indeed, the majority of plant chitinases consist of at most only one chitin-binding domain and a catalytic domain.7 Also, the removal of one or both chitin-binding domains in recombinant BjCHI1 did not adversely affect chitinase activity of BjCHI1 when expressed in P. pastoris.6
Conclusions: Dual Functions of BjCHI1 in Plant Defense
Chitinases are a group of important defense-related proteins in plants. Our previous studies have shown that expression of BjCHI1 mRNA is induced by wounding, methyl jasmonate,1 Aspergillus niger infection and feeding by caterpillars of Pieris rapae.4 Transgenic tobacco4 and transgenic potato5 extracts demonstrated anti-fungal activity against Trichoderma viride and transgenic potato plants were conferred protection against the soil-borne pathogen, Rhizoctonia solani.5
Based on the absence of anti-bacterial activity in BjCHI1 derivatives lacking the chitin-binding domains, the bivalent structure of the carbohydrate-binding domains in BjCHI1 appears essential in defense against bacteria. The formation of two- and three-dimensional supermolecular assemblies13 from the interaction of the lipopolysaccharide components, present on the outer membranes of bacterial cell walls, in conjunction with BjCHI1, would immobilize bacteria. Further, the second chitin-binding domain may confer a synergistic effect on BjCHI1 with other chitinases, as well as in enhancing its own ability to access reactive sites on fungal hyphae. Our study provides evidence in an evolution of a multi-functional protein within the chitinase family.
Chitinases are presumably safer pesticides than chemically-synthesized alternatives. In agriculture, such chitinolytic enzymes are important in controlling phytopathogenic fungi, insects and nematodes. This study provides evidence on the versatility of BjCHI1 in defense against both pathogenic bacteria, including R. solanacearum (causative agent of bacterial wilt), as well as against fungal phytopathogens, C. truncatum and C. acutatum (causative agents of anthracnose affecting fruit and plantation crops), and B. cinerea (causative agent of blight). That such protection could be rendered in transplastomic tobacco suggests some promise for BjCHI1 in genetic engineering for disease-resistance, despite limitations14 in the use of such strategies due to complexities arising from biological systems.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7006
References
- 1.Zhao KJ, Chye ML. Methyl jasmonate induces expression of a novel Brassica juncea chitinase with two chitin-binding domains. Plant Mol Biol. 1999;40:1009–1018. doi: 10.1023/a:1006266407368. [DOI] [PubMed] [Google Scholar]
- Beintema JJ, Peumans WJ. The primary structure of stinging nettle (Urtica dioica) agglutinin, a two-domain member of the hevein family. FEBS Letts. 1992;299:131–134. doi: 10.1016/0014-5793(92)80231-5. [DOI] [PubMed] [Google Scholar]
- 3.Lerner DR, Raikhel NV. The gene for stinging nettle lectin (Urtica dioica agglutinin) encodes both a lectin and a chitinase. J Biol Chem. 1992;267:11085–11091. [PubMed] [Google Scholar]
- 4.Fung KL, Zhao KJ, He ZM, Chye ML. Tobacco-expressed Brassica juncea chitinase BjCHI1 shows antifungal activity in vitro. Plant Mol Biol. 2002;50:283–294. doi: 10.1023/a:1016067200148. [DOI] [PubMed] [Google Scholar]
- 5.Chye ML, Zhao KJ, He ZM, Ramalingam S, Fung KL. Expression of an agglutinating chitinase BjCHI1 with two chitin-binding domains is R. solani-inducible and confers fungal protection in transgenic potato. Planta. 2005;220:717–730. doi: 10.1007/s00425-004-1391-6. [DOI] [PubMed] [Google Scholar]
- 6.Tang CM, Chye ML, Ramalingam S, Ouyang SW, Zhao KJ, Ubhayasekera W, Mowbray S. Functional analysis of the chitin-binding domains and the catalytic domain of Brassica juncea chitinase BjCHI1. Plant Mol Biol. 2004;56:285–298. doi: 10.1007/s11103-004-3382-1. [DOI] [PubMed] [Google Scholar]
- 7.Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K. Plant chitinases. Plant J. 1993;3:31–40. doi: 10.1046/j.1365-313x.1993.t01-1-00999.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Parijs J, Broekaert WF, Goldstein IJ, Peumans WJ. Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta. 1991;183:258–264. doi: 10.1007/BF00197797. [DOI] [PubMed] [Google Scholar]
- 9.Wright HT, Sandrasegaram G, Wright CS. Evolution of a family of N-acetylglucosamine binding proteins containing the disulfide-rich domain of wheat germ agglutinin. J Mol Evol. 1991;33:283–294. doi: 10.1007/BF02100680. [DOI] [PubMed] [Google Scholar]
- 10.Peumans WJ, Van Damme EJM. Lectins as plant defense proteins. Plant Physiol. 1995;109:347–352. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Damme EJMV, Peumans WJ, Pusztai A, Bardocz S. Handbook of plant lectins: properties and biomedical applications. Chichester: John Wiley and Sons; 1998. [Google Scholar]
- 12.Beintema JJ. Structural features of plant chitinases and chitin-binding proteins. FEBS Letts. 1994;350:159–163. doi: 10.1016/0014-5793(94)00753-5. [DOI] [PubMed] [Google Scholar]
- 13.Brewer CF. Cross-linking activities of galectins and other multivalent lectins. Trends Glycosci Glycotech. 1997;9:155–165. [Google Scholar]
- 14.Collinge DB, Lund OS, Thordal-Christensen H. What are the prospects for genetically engineered, disease resistant plants? Eur J Plant Pathol. 2008;121:217–231. [Google Scholar]