Abstract
Cannabis sativa is well known to produce unique secondary metabolites called cannabinoids. We recently discovered that Cannabis leaves induce cell death by secreting tetrahydrocannabinolic acid (THCA) into leaf tissues. Examinations using isolated Cannabis mitochondria demonstrated that THCA causes mitochondrial permeability transition (MPT) though opening of MPT pores, resulting in mitochondrial dysfunction (the important feature of necrosis). Although Ca2+ is known to cause opening of animal MPT pores, THCA directly opened Cannabis MPT pores in the absence of Ca2+. Based on these results, we conclude that THCA has the ability to induce necrosis though MPT in Cannabis leaves, independently of Ca2+. We confirmed that other cannabinoids (cannabidiolic acid and cannabigerolic acid) also have MPT-inducing activity similar to that of THCA. Moreover, mitochondria of plants which do not produce cannabinoids were shown to induce MPT by THCA treatment, thus suggesting that many higher plants may have systems to cause THCA-dependent necrosis.
Key words: cannabinoid, Cannabis sativa, cylophilin D, mitochondrial permeability transition, necrosis
Cannabis sativa produces unique secondary metabolites consisting of alkylresorcinol and monoterpene groups.1 These metabolites called cannabinoids are well known to show a variety of interesting pharmacological activities including psychoactive effect and analgesic effect. Therefore, cannabinoids have attracted a great deal of attention, whereas why C. sativa produces such metabolites has long remained unclear. However, we have recently obtained evidences indicating the physiological function of THCA in Cannabis leaves.2
We discovered that THCA is stored in capitate-sessile glands on Cannabis leaves and that secretion of this cannabinoid into leaf tissues causes cell death. When the properties of THCA were examined using cultured Cannabis cells, this cannabinoid induced plasmamembrane shrinkage and DNA degradation. These responses are regarded as the features of apoptotic cells, but were not suppressed by apoptosis inhibitors. In contrast, the necrosis inhibitor cyclosporine A significantly inhibited both plasmamembrane shrinkage and DNA degradation in Cannabis cells. Therefore, we assumed that THCA induces necrotic cell death in Cannabis cells and leaves.
Necrosis in plants and animals is usually triggered by MPT though opening of MPT pores.3,4 MPT is known to cause mitochondrial dysfunction by mitochondrial swelling and loss of mitochondrial membrane potential (ΔΨm),5,6 and we also confirmed that THCA induces mitochondrial swelling and ΔΨm reduction in mitochondria isolated from Cannabis cells and that pretreatment with cyclosporine A inhibits both responses. Based on these evidences, we concluded that THCA has the activity to induce MPT-dependent necrosis.
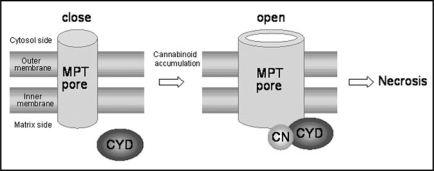
As described above, MPT pores play an important role in necrosis induction, whereas the mechanism of their opening in higher plants has not been fully understood. However, binding of cyclophilin D (a protein present in mitochondrial matrix) to MPT pores is shown to be essential for their opening in plants as well as animal.7–9 In animal mitochondria, Ca2+ mediates this binding reaction, leading to opening of MPT pores. Wheat mitochondria are also shown to undergo swelling through opening of MPT pores in response to Ca2+,9 whereas MPT pores of oats,10 Arabidopsis thaliana11 and C. sativa2 do not open by Ca2+ treatment. In contrast, THCA catalyzed opening of Cannabis MPT pores in the absence of Ca2+, suggesting that THCA directly mediates binding of cyclophilin D to MPT pores (Fig. 1). In addition, we have now confirmed that THCA causes dysfunction though MPT in mitochondria of plants (rice, soybean, A. thaliana and Scutellaria baicalensis) lacking cannabinoid-producing ability (data not shown). Therefore, many higher plants may have the systems to induce THCA-dependent necrosis.
Figure 1.
A model depicting the opening mechanism of MPT pores in mitochondria. CYD, cyclophilin D; CN, cannabinoid.
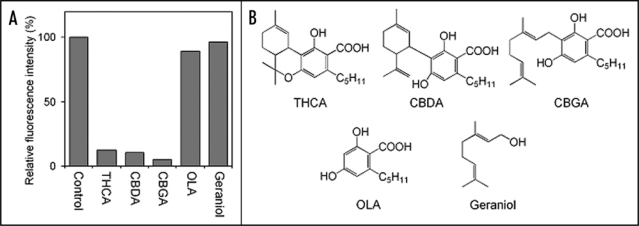
Furthermore, we investigated whether other cannabinoids and their related compounds can mediate MPT in Cannabis mitochondria. When the MPT-inducing activity of each sample was measured by monitoring both ΔΨm reduction (Fig. 2) and mitochondrial swelling (data not shown), we confirmed that cannabinoids tested here (cannabidiolic acid and cannabigerolic acid) possess the activities similar to those of THCA. On the other hand, olivetolic acid (the akylresorcinol moiety of cannabinoid) and geraniol (the monoterpene moiety of cannabigerolic acid) showed neither ΔΨm reduction nor mitochondrial swelling (Fig. 2). These results suggested that the structures (cannabinoid skeleton) where monoterpene and olivetolic acid are coupled to each other seem essential for opening of MPT pores. Therefore, we assumed that plant cyclophilin D and MPT pores have the cannabinoid-binding site.
Figure 2.
Change of ΔΨm by treatment with various compounds (A) and their chemical structures (B). The isolated mitochondria were stained with the ΔΨm-indicating reagent (tetramethylrhodamine methylester, TMRM) and then incubated with 200 µM of each compound for 60 min. The intensity of TMRM fluorescence was measured using a fluorescence microplate reader. A decrease of the fluorescence intensity indicates ΔΨm reduction. CBDA, cannabidiolic acid; CBGA, cannabigerolic acid; OLA, olivetolic acid.
Plant cell death is shown to participate in important physiological responses such as leaf senescence, somatic embryogenesis and defense against microbial pathogens.12,13 Based on its induction mechanism, plant cell death is largely classified into apoptosis and necrosis. Although the molecular mechanism of apoptosis has been extensively investigated, there is little precise information on plant necrosis. However, our study would provide important insight into necrosis-inducing mechanisms in higher plants.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7011
References
- 1.Mechoulam R. Marihuana chemistry. Science. 1970;168:1159–1166. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto S, Tanaka Y, Sasaki K, Tanaka H, Fukamizu T, Shoyama Y, Shoyama Y, Taura F. Identification and characterization of cannabinoids that induce cell death through mitochondrial permeability transition in Cannabis leaf cells. J Biol Chem. 2007;282:20739–20751. doi: 10.1074/jbc.M700133200. [DOI] [PubMed] [Google Scholar]
- 3.He LH, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 4.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–518. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 6.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;3005:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 7.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 9.Arpagaus S, Rawyler A, Braendle R. Occurrence and characteristics of the mitochondrial permeability transition in plants. J Biol Chem. 2002;277:1780–1787. doi: 10.1074/jbc.M109416200. [DOI] [PubMed] [Google Scholar]
- 10.Curtis MJ, Wolpert TJ. The oat mitochondrial permeability transition and its implication in victorin binding and induced cell death. Plant J. 2002;29:295–312. doi: 10.1046/j.0960-7412.2001.01213.x. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari BS, Belenghi B, Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002;128:1271–1281. doi: 10.1104/pp.010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg JT. Programmed cell death: A way of life for plants. Proc Natl Acad Sci USA. 1996;93:12094–12097. doi: 10.1073/pnas.93.22.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennell RI, Lamb C. Programmed cell death in plants. Plant Cell. 1997;9:1157–1168. doi: 10.1105/tpc.9.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]