Abstract
Extracellular Cu/Zn superoxide dismutases (CSDs) that catalyze the conversion of superoxide to hydrogen peroxide have been suggested to be involved in lignification of secondary walls in spinach, pine and aspen. In cotton fibers, hydrogen peroxide was proposed to be involved in the induction of secondary cell wall biosynthesis. Recently, we identified extracellular CSDs from developing cotton fibers using both immunological and epitope tagging techniques. Since cotton fibers are not lignified, we suggested that extracellular CSDs may be involved in plant cell wall growth and development processes other than lignification. In this addendum, we have further characterized the extracellular CSD in cotton fiber. Immunoblots, enzyme activity assays, and transcript levels show that an extracellular CSD is present in elongating primary walls as well as thickening secondary walls of cotton fibers. Our working model proposes that extracellular hydrogen peroxide levels, regulated by redox status-related enzymes including extracellular CSDs and peroxidases, may affect the processes of wall loosening and wall tightening.
Key words: cell wall, cotton fibers, Cu/Zn superoxide dismutase, Gossypium hirsutum, hydrogen peroxide, reactive oxygen species
Reactive oxygen species (ROS) are now attributed as major signaling molecules in aerobic organisms, including plants. In order for plants to use these potentially toxic molecules as signals requires that a relatively large network of genes must have evolved to control the production and scavenging of ROS.1 In Arabidopsis, for example, this ‘reactive oxygen network’ is composed of more than 150 genes whose products are localized in nearly every subcellular compartment. Although many of the enzymes involved in ROS production and detoxification that are localized in chloroplasts, mitochondria, peroxisomes, and the cytosol have been thoroughly studied, similar functions in the apoplast/cell wall have not been well characterized.
Superoxide (O2−) and hydrogen peroxide (H2O2) play important roles in the covalent crosslinking between protein and carbohydrate cell wall components and in lignin biosynthesis.2,3 The conversion of O2− to H2O2 is catalyzed by superoxide dismutases (SODs). The first type of SOD shown to localize to cell walls was a Cu/Zn-type SOD (CSD) from spinach mesophyll cells.4 Additional extracellular (EC) CSDs have been identified from pine and aspen.5,6 Immunolocalization studies showed that EC CSDs localized to the secondary cell wall (SCW) and intercellular spaces of pine, and to lignified SCWs of phloem fibers and xylem vessels of aspen.5,7
Due to the predominant localization of EC CSDs to SCWs, the principal function of these enzymes has been proposed to be cell wall lignification;4–6 however, the unexpected dwarf phenotypes of transgenic hybrid aspen in which EC CSD expression was reduced by antisense techniques raises the question of whether EC CSDs are merely involved in SCW biosynthesis.7 Reduced cell division and expansion of the severely dwarfed plants suggests that EC CSDs may be involved in primary cell wall (PCW) biosynthesis for expansion. Thus, the physiological functions of EC CSDs are unclear at present.
To elucidate the physiological function of EC CSDs in plant wall biosynthesis, we used cotton fibers as a model system. Cotton (Gossypium hirsutum, L.) fibers are unicellular trichomes arising from the epidermis of developing cotton ovules. Fibers elongate up to 3∼6 cm for 2–3 weeks after anthesis. For approximately the first 2 weeks, fiber cells are delimited by only a PCW. SCW biosynthesis initiates approximately 14 to 16 days post anthesis (DPA) and continues until approximately 45 DPA or longer.8 As a result, unlike most multicellular organisms in which different cell types may be at different developmental or cell division stages, cotton fiber PCW and SCW biosynthesis can be monitored independently by selecting fibers of the appropriate age.8
Hydrogen peroxide has been proposed to be involved in the induction of SCW cellulose biosynthesis and dimerization of cellulose synthase subunits during cotton fiber development.9,10 In developing cotton fibers, we have identified three groups of CSDs and determined the subcellular localization of their products by both immunological and epitope tagging techniques.11 Among the three types of CSDs, only GhCSD3, tagged with green fluorescent protein (GFP) or c-myc, translocated to cell walls despite the absence of a signal peptide. GhCSD1 localized to the cytosol, and GhCSD2 localized to plastids. Unlike GhCSD1 and GhCSD2 that were predominantly expressed in elongating tissues such as hypocotyls, young roots, and elongating fibers, GhCSD3 was highly expressed in older tissues like fully expanded leaves.11 Since cotton fiber SCWs are not lignified, we proposed that EC GhCSD3 may be involved in plant cell wall growth and development processes other than lignification.
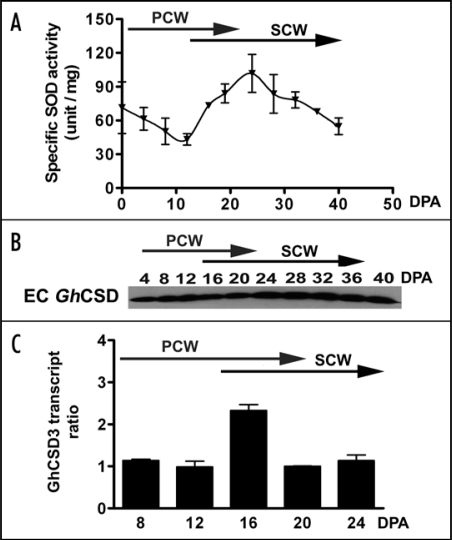
In this addendum, we present data supporting the notion that EC GhCSD3 is involved in both PCW and SCW biosynthesis in developing cotton fibers. We have further characterized EC GhCSD3 accumulation throughout fiber development. Enzyme activity measurements (Fig. 1A) and an immunoblot (Fig. 1B) were conducted with extracellular proteins extracted from fiber-bearing cotton seeds. SOD activity was detected throughout development, declining from 0–10 DPA and peaking again at ∼25 DPA (Fig. 1A). Immunoblot analysis confirmed that a CSD was present in these extracellular protein extracts (Fig. 1B). The enzyme activity measurements and immunoblot are composite analyses of EC SODs from seed and fiber, so as to avoid contaminating the preparations with cytoplasmic or organellar forms of the enzyme. In contrast, transcript abundance can be independently assessed in fiber cells. GhCSD3 transcripts were analyzed by quantitative, reverse transcription PCR (Q-RT-PCR) using RNAs from detached fibers (Fig. 1C). Although GhCSD3 transcript abundance increased at the transition to SCW, GhCSD3 is clearly expressed throughout fiber development. Furthermore, GhCSD3 tagged with GFP translocated to PCWs of Arabidopsis roots.11 As a result, we suggest here that EC GhCSD3 is associated with biosynthesis of the PCW as well as the SCW.
Figure 1.
(A) SOD specific activity assay of EC proteins isolated from DOA-40 DPA seeds. Extracellular proteins were extracted from trichome-bearing cotton seeds using 1 M NaCl and assayed for SOD activity according to the method of Flohè and Ötting.17 PCW, primary cell wall stage; SCW, secondary cell wall stage. (B) Immunoblot analysis of GhCSD extracted from cotton seeds from 4–40 DPA using a plant CSD antibody. EC protein was separated on a 15% SDS-polyacrylamide gel and blotted to nitrocellulose. Primary antibody was anti-plant CSD (1:6000 dilution) (EnVirtue Biotechnologies Inc., Winchester, VA). Secondary antibody was horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:2000 dilution) with detection by SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). (C) Relative transcript abundance of GhCSD genes in fiber. Relative transcript abundance of GhCSD genes in 8-24 DPA fibers measured by Q-RT-PCR. Specific primers for GhCSD3 (5′-CCATGCTGGAGATT TGGGTA-3′/ 5′-TCAGCAAC CCATCAGGGC-3′) and cotton 18S rRNA (5′-CGTCCCTGCCCTTTGTACA-3′/5′-AACCTTCACCGGACCATTCA-3′) as a normalizer were designed using Primer Express software (version 2.0, Applied Biosystems, Foster City, CA). The specificity of primer annealing was examined by monitoring product dissociation. The fold difference is relative to the lowest transcript level present in 12 DPA fibers for GhCSD3.
The presence of GhCSD3 activity throughout fiber development suggests that H2O2 is produced during both PCW and SCW biosynthesis. High levels of H2O2 and metal ions (30–90 ppm of iron, 1–10 ppm of Zn and Cu) in elongating fiber cell walls are favorable for the production of hydroxyl radical through the Fenton reaction that can loosen the cell walls for elongation.12–14 Levels of H2O2 produced by GhCSD3 could be controlled by wall-associated ROS scavenging enzymes that use hydrogen peroxide as a substrate. Alternatively, due to the unrestricted movement of H2O2 within cells, enzymes in other cellular compartments may detoxify this ROS. It is noteworthy that a recent study identifies a cytosolic ascorbate peroxidase as an enzyme that accumulates to high levels during fiber elongation.15 Similarly, comparative gene expression profiling between two cotton species that differ in their production of long, spinnable fibers revealed significant differences in the expression of genes related to ROS regulation.16
In summary, the presence of EC GhCSD3 in elongating PCW and thickening SCW in cotton fibers suggests that H2O2 is involved in the biogenesis of both PCW and SCW. Our working model proposes that the level of extracellular H2O2, regulated by redox status-related enzymes including EC GhCSD3 and other enzymes, may affect the processes of wall loosening and wall tightening.
Acknowledgements
This work was supported by USDA-ARS, NASA, NSF and the Louisiana State Support Program of Cotton Incorporated. We thank Yong-Mei Qin of Peking University, Jay Mellon and Jay Shockey of USDA-ARS-SRRC for critically reading the manuscript.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7039
References
- 1.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trend Plant Sci. 2004;9:1360–1368. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Kieliszewski JJ, Lamport DTA. Extensin: repetitive motifs, functional sites, post-translational codes and phylogeny. Plant J. 1994;5:157–172. doi: 10.1046/j.1365-313x.1994.05020157.x. [DOI] [PubMed] [Google Scholar]
- 3.Pomar F, Caballero N, Pedreno M, Ros Barcelo A. H2O2 generation during the auto-oxidation of coniferyl alcohol drives the oxidase activity of a highly conserved class III peroxidase involved in lignin biosynthesis. FEBS Lett. 2002;529:198–202. doi: 10.1016/s0014-5793(02)03339-2. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa K, Kanematsu S, Asada K. Intra-and extra-cellular localization of cytosolic CuZn-superoxide dismutase in spinach leaf and hypocotyls. Plant Cell Physiol. 1996;37:790–799. [Google Scholar]
- 5.Karpinska B, Karlsson M, Schinkel H, Streller S, Süss KH, Melzer M, Wingsle G. A novel superoxide dismutase with a high isoelectric point in higher plants; expression, regulation and protein localisation. Plant Physiol. 2001;126:1668–1677. doi: 10.1104/pp.126.4.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schinkel H, Hertzberg M, Wingsle G. A small family of novel CuZn-superoxide dismutases with high isoelectric points in hybrid aspen. Planta. 2001;213:272–279. doi: 10.1007/s004250000505. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava V, Schinkel H, Witzell J, Hertzberg M, Torp M, Srivastava MK, Karpinska B, Melzer M, Wingsle G. Downregulation of high-isoelectric-point extracellular superoxide dismutase mediates alterations in the metabolism of reactive oxygen species and developmental disturbances in hybrid aspen. Plant J. 2007;49:135–148. doi: 10.1111/j.1365-313X.2006.02943.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Triplett BA. Cotton fiber growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant Physiol. 2001;127:1361–1366. [PMC free article] [PubMed] [Google Scholar]
- 9.Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999;119:849–858. doi: 10.1104/pp.119.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurek I, Kawagoe Y, Jacob-Wilk D, Doblin M, Delmer D. Dimerization of cotton fiber cellulose synthase catalytic subunits occurs via oxidation of the zinc-binding domains. Proc Natl Acad Sci USA. 2002;99:11109–11114. doi: 10.1073/pnas.162077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Kato N, Kim S, Triplett B. Cu/Zn superoxide dismutases in developing cotton fibers: Evidence for an extracellular form. Planta. 2008;228:281–292. doi: 10.1007/s00425008-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakelyn PJ, Bertoniere NR, French AD, Thibodeaux DP, Triplett BA, Rousselle MA, Goynes WR, Jr, Edwards JV, Hunter L, McAlister DD, Gamble GR. Cotton fiber chemistry and technology. Boca Raton, FL: CRC Press; 2007. p. 162. [Google Scholar]
- 13.Chen SX, Schopfer P. Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. Eur J Biochem. 1999;260:726–735. doi: 10.1046/j.1432-1327.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- 14.Schweikert C, Liszkay A, Schopfer P. Scission of polysaccharides by peroxidase-generated hydroxyl radicals. Phytochemistry. 2000;53:565–570. doi: 10.1016/s0031-9422(99)00586-5. [DOI] [PubMed] [Google Scholar]
- 15.Li H-B, Qin Y-M, Pang Y, Song W-Q, Mei W-Q, Zhu U-X. A cotton ascorbate peroxidase is involved in hydrogen peroxide homeostasis during fibre cell development. New Phytol. 2007;175:462–471. doi: 10.1111/j.1469-8137.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 16.Hovav R, Udall JA, Chaudhary B, Hovav E, Flagel L, Hu G, Wendel JF. The evolution of spinnable cotton fiber entailed prolonged development and a novel metabolism. PLOS Genetics. 2008;4:25. doi: 10.1371/journal.pgen.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flohè L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]