Abstract
Polarity is a fundamental cell property essential for differentiation, proliferation and morphogenesis in unicellular and multicellular organisms. We have recently demonstrated that phosphatidylinositol 3-kinase (PI3K) activity is required for the establishment of anterior-posterior axis, leading to asymmetrical localization of F-actin in migrating monospores of the red alga Porphyra yezoensis. We also showed that the formation of the apical-basal axis via adhesion of monospores to the substratum after the cessation of migration requires newly synthesized proteins and does not depend on PI3K activity. However, little is known about the mechanism and regulation of axis conversion during development of monospores. In this addendum, we report our investigation as to the role of the cell wall in axis conversion. Our results indicate that inhibition of cell wall synthesis prevented the development of germlings. Also, defects in the cell wall disrupted the asymmetrical distribution of F-actin and inhibited the adhesion to the substratum that is required for establishment of apical-basal axis. Hence, we conclude that the cell wall is critical for the maintenance of cell polarity in migrating cells, which is indirectly involved in axis conversion via enabling monospores to adhere to the substratum.
Key words: BFA, cell polarity, cell wall, F-actin, monospores, PI3K, Porphyra yezoensis
The initial establishment of cell polarity, which is exhibited in asymmetrical cell division and directional migration, depends on asymmetrical cues that lead to reorganization of the cytoskeleton and polarized distribution of cortical proteins and membrane lipids.1–3 For directional migration of Dictyostelium cells and leukocytes, cells in the axialized form can rapidly change their body shape along with the formation of cell polarity in response to external impulses such as cAMP and cytokines, enabling them to migrate toward the external impulse with driving and contractile forces provided by asymmetrically distributed cytoskeletal elements.4,5 Evidence is growing that in both asymmetrical cell division and migration, intracellular compartmentalization of phosphatidylinositol 3-kinase (PI3K) and phosphatidylinositol polyphosphate phosphatases is responsible for the asymmetrical and reciprocal distributions of PI(3,4,5)P3 and PI(4,5)P2 on plasma membranes. This helps cells to define their polarity by organizing polarized localization of F-actin and myosin.6–8
We used the monospores of the red alga Porphyra yezoensis to elucidate the molecular mechanisms involved in the establishment of cell polarity in plants. Migration and asymmetrical cell division are both observed during the early development of monospores released from monosporangia produced at the marginal region of the thallus.9,10 Thus, monospores are thought to be unique and useful materials for investigating polarity determination in plant cells. In the early development of monospores, there are two different cellular axes: the anterior-posterior axis during migration and the apical-basal axis in asymmetrical cell division and upward growth of a thallus. The use of LY294002, a PI3K inhibitor, prevented the migration of monospores because the anterior-posterior axis cannot be established. This is evidence that the PI3K activity is essential for the establishment of cell polarity and asymmetric distribution of F-actin for migration of monospores.10 Thus, the formation of the former axis requires PI3K activity and asymmetrical distribution of F-actin.10 These results are similar to those observed in Dictyostelium cells and leukocytes, suggesting that the role of PI3K-dependent F-actin asymmetry in the establishment of cell polarity might be evolutionarily conserved in migrating eukaryotic cells. However, it is still unclear whether PI(3,4,5)P3 corresponds to D3-phosphorylated phosphatidylinositol in P. yezoensis, since this phosphoinositide has not yet been detected in any plant cell.11,12
In addition to D3-phosphorylated phosphatidylinositols, it is well known that the cell wall plays an essential role in the establishment of cell polarity, a phenomenon documented in the brown algae Fucus.13–15 It has been demonstrated that the cell wall is required for the fixation, but not the formation, of the apical-basal axis.13,14 In this case, polarized secretion via Golgi apparatus is needed for synthesis of the cell wall.15 In fact, we observed that monospores whose migration was inhibited by treatment with PI3K and cytoskeleton inhibitors have no cell wall,10 suggesting the importance of the cell wall in formation and/or maintenance of the cell axis in P. yezoensis.
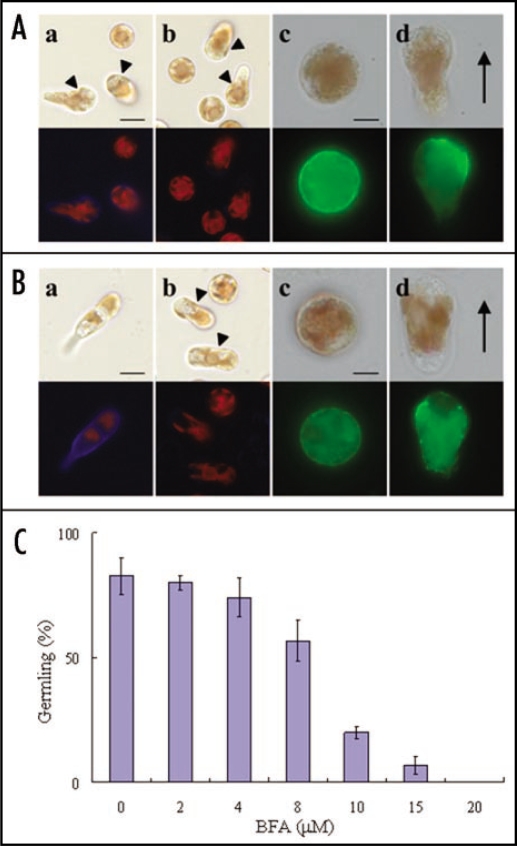
To confirm this possibility, we used Brefeldin A (BFA), a specific inhibitor of polysaccharide biosynthesis required for cell wall formation via Golgi-derived vesicle trafficking. As shown in Figure 1A, part a, the cell wall was synthesized during migration of monospores. However, when freshly released monospores were treated with BFA for 3 h, there was no cell wall synthesis in monospores with a rounded shape or in migrating monospores with a tapered shape (Fig. 1A, part b). This evidence led us to conclude that Golgi-derived vesicle trafficking is responsible for cell wall formation in these monospores. These results also indicated that the anterior-posterior axis during migration can be established without cell wall synthesis. Indeed, F-actin accumulated at the leading edge in the migrating monospores in the presence of BFA (Fig. 1A, part d).
Figure 1.
Effect of BFA on the cell wall synthesis, F-actin localization and development of monospores. (A) Freshly released monospores were treated with (parts b–d) or without (part a) BFA (MP Biomedicals) at 20 µM for 3 h incubation. The cell wall (parts a and b) and F-actin (parts c and d) were stained with 0.01% Fluorescent Brightener 28 (Sigma) and 5 U· mL−1 Alex Flour 488 phalloidin (Molecular probe), respectively. Migrating monospores are indicated by arrowheads. (Part c) Cell with a round shape, (Part d) cell with a tapered shape during migration. Upper and lower photographs in each panel show bright field and fluorescent images, respectively. Direction of migrating monospores is indicated by an arrow. Scale bars: (Parts a and b) 10 µm; (Parts c and d) 5 µm. (B) Freshly released monospores were treated with (parts b–d) or without (part a) BFA at 20 µM for 24 h incubation. The cell wall (parts a and b) and F-actin (parts c and d) were stained with 0.01% Fluorescent Brightener 28 and 5 U· mL−1 Alex Flour 488 phalloidin, respectively. Migrating monospores are indicated by arrowheads. (Part c) Cell with a round shape, (Part d) cell with a tapered shape during migration. Upper and lower photographs in each panel show bright field and fluorescent images, respectively. Direction of migrating monospores is indicated by an arrow. Scale bars: (Parts a and b) 10 µm; (Parts c and d) 5 µm. (C) Dose-dependent effect of BFA on early development of monospores. Freshly released monospores were treated with an increasing concentration (2–20 µM) of BFA for 24 h, and the number of germlings was counted. Data are presented as mean ± SD (n = 3).
Next, we analyzed the relationship between cell wall synthesis and development of germlings to confirm the functional significance of cell wall synthesis in the establishment of apical-basal axis. In the control medium, the cell wall was observed in 2-celled germlings 24 h after monospores release (Fig. 1B, part a), while the BFA-treated monospores still retained the migrating form in which the cell wall was not synthesized (Fig. 1B, part b) and the adhesion of monospores to the substratum and development of germlings was prevented (Fig. 1B, parts b–d). Moreover, the rate of the development of germlings from monospores decreased in a dose-dependent manner after 24 h incubation with BFA (Fig. 1C). Notably, it is significant that the polarized localization of F-actin in migrating cells was destroyed during BFA treatment (Fig. 1B, part d), suggesting the involvement of the cell wall in the maintenance of the asymmetrical distribution of F-actin in migrating monospores. Thus, the cell wall is indispensable for maintenance of anterior-posterior axis in migrating cells. Moreover, since adhering is trigger of the formation of the apical-basal axis, synthesis of cell wall could enable cells to develop further into germlings. We therefore concluded that cell wall plays a role indirectly in axis conversion during the development of monospores. Future work should be focused on the nature of the cell wall factors involved in the maintenance of cell axis and the adhesion to the substratum and how the function and expression of these factors are regulated.
In summary, the establishment and maintenance of cell polarity during development of monospores is under complex regulation. Dissecting the molecular mechanisms of this regulatory system could help in further understanding the interrelation among PI3K signaling, the actin-based system and cell wall formation, which can provide new insight into the machinery regulating the establishment and maintenance of cell polarity in plants.
Acknowledgements
We are grateful to Dr. Hajime Yasui (Hokkaido University, Japan) for kindly providing the microscopes. This study was supported in part by a grant from the Sumitomo Foundation (to K.M.) and by Grants-in-Aid for the 21st COE (Center Of Excellence) Program ‘Marine Bio-Manipulation Frontier for Food Production’ and the City Area Program in Industry-Academia-Government Joint Research (Hakodate area) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to N.S.).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/7039
References
- 1.Way JC, Wang L, Run JQ, Hung MS. Cell polarity and the mechanism of asymmetric cell division. BioEssays. 1994;16:925–931. doi: 10.1002/bies.950161212. [DOI] [PubMed] [Google Scholar]
- 2.Williams HP, Harwood AJ. Cell polarity and Dictyostelium development. Curr Opin Microbiol. 2003;6:621–627. doi: 10.1016/j.mib.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Gomez TS, Billadeau DD. T Cell activation and the cytoskeleton: you can't have one without the other. Adv Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 4.Harris SJ, Parry RV, Westwick J, Ward SG. Phosphoinositide lipid phosphatases: natural regulators of phosphoinositide 3-kinase signaling in T lymphocytes. J Biol Chem. 2008;283:2465–2469. doi: 10.1074/jbc.R700044200. [DOI] [PubMed] [Google Scholar]
- 5.Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourne HR, Weiner O. Cell polarity: A chemical compass. Nature. 2002;419:21. doi: 10.1038/419021a. [DOI] [PubMed] [Google Scholar]
- 7.Iijima M, Huang YE, Devreotes P. Temporal and spatial regulation of chemotaxis. Dev Cell. 2002;3:469–478. doi: 10.1016/s1534-5807(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 8.Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–347. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Hawkes MW. Ultrastructure characteristics of monospores formation in Porphyra gardneri (Rhodophyta) J Phycol. 1980;16:192–196. [Google Scholar]
- 10.Li L, Saga N, Mikami K. Phosphatidylinositol 3-kinase activity and asymmetrical accumulation of F-actin are necessary for establishment of cell polarity in the early development of monospores from the marine red alga Porphyra yezoensis. J Exp Bot. 2008;59:3575–3586. doi: 10.1093/jxb/ern207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller-Roeber B, Pical C. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoform of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol. 2002;130:22–46. doi: 10.1104/pp.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zonia L, Munnik T. Cracking the green paradigm: functional coding of phosphoinositide signals in plant stress responses. Subcell Biochem. 2006;39:207–238. doi: 10.1007/0-387-27600-9_9. [DOI] [PubMed] [Google Scholar]
- 13.Kropf DL, Kloareg B, Quatrano RS. Cell wall is required for fixation of the embryonic axis in Fucus zygotes. Science. 1988;239:187–190. doi: 10.1126/science.3336780. [DOI] [PubMed] [Google Scholar]
- 14.Fowler JE, Quatrano RS. Plant cell morphogenesis: plasma membrane interactions with the cytoskeleton and cell wall. Annu Rev Cell Dev Biol. 1997;13:697–743. doi: 10.1146/annurev.cellbio.13.1.697. [DOI] [PubMed] [Google Scholar]
- 15.Belanger KD, Quatrano RS. Polarity: the role of localized secretion. Curr Opin Plant Biol. 2000;3:67–72. doi: 10.1016/s1369-5266(99)00043-6. [DOI] [PubMed] [Google Scholar]