Abstract
Plants regulate their growth and morphogenesis in response to gravity field, known as gravitropism. In the early process of gravitropism, changes in the gravity vector (gravistimulation) are transduced into certain intracellular signals, termed gravity perception. The plant hormone auxin is not only a crucial factor to represent gravitropism but also a potential signaling molecule for gravity perception. Another strong candidate for the signaling molecule is calcium ion of which cytoplasmic concentration ([Ca2+]c) is known to increase in response to gravistimulation. However, relationship between these two factors, say which is in the first place, has been controversial. This issue is addressed here mainly based on recent progress including our latest studies. Gravistimulation by turning plants 180° induced a two-peaked [Ca2+]c-increase lasting for several minutes in Arabidopsis seedlings expressing apoaequorin; only the second peak was sensitive to the gravistimulation. Peak amplitudes of the [Ca2+]c-increase were attenuated by the 10 µM auxin transport inhibitor (TIBA) and vesicle trafficking inhibitor (BFA), whereas the onset time and rate of rise of the second peak were not significantly altered. This result indicates that polar auxin transport is not involved in the initial phase of the second [Ca2+]c-increase. It is likely that the gravi-induced [Ca2+]c-increase constitutes an upstream event of the auxin transport, but may positively be modulated by auxin since its peak amplitude is attenuated by the inhibition of auxin transport.
Key words: auxin, calcium, gravity perception, gravitropism, pin-formed (PIN) protein, Arabidopsis thaliana
Signaling Molecules Involved in Plant Gravitropism
Higher plants sense gravity and orient their growth direction with respect to the gravity vector. In general, shoots (e.g., coleoptiles, hypocotyls and inflorescence stems) grow upwards and roots grow downwards even in darkness, a phenomenon known as gravitropism.1 Although the gravitropic morphological changes have been well documented, mechanisms of gravity perception and following signal transduction remain largely obscure. In the process of gravity perception, changes in the gravity vector (gravistimulation) will be transduced into multiple intracellular signals, which has been investigated since the days of Charles Darwin.2 The plant hormone auxin was identified as one of the signaling molecules and its redistribution has been thought to be involved in gravitropic responses, known as Cholodny-Went theory.3 This theory proposes that asymmetrical distribution of auxin causes differential growth, resulting in directional bending of organs. In fact, gravistimulation induces an asymmetrical auxin distribution in gravitropic organs such as tobacco stems,4 tomato hypocotyls,5 Arabidopsis hypocotyls6 and roots,7,8 which is consistent with this theory. The asymmetrical auxin distribution appears to be established by lateral and polar transport of auxin during gravistimulation. However, the underlying molecular and cellular mechanisms had been unknown for a long time.
Recent genetic analyses in Arabidopsis have clarified molecules responsible for the auxin transport and its up- and downstream signals.9 Pin-formed (PIN) proteins were characterized as an auxin efflux regulator10,11 and their localization plays an important role in determining the direction of auxin flux.6,12 Gravistimulation induces translocation of PIN3 with respect to the gravity vector in columella cells, possibly causing the polar auxin transport toward the bottom side of horizontally placed Arabidopsis roots.6,13 Molecules mediating the localization of PIN proteins have also been identified in Arabidopsis such as GNOM,14,15 a guanine-nucleotide exchange factor on ADP-ribosylation factor G protein (ARF GEF) and PINOID (PID),16 a Ser/Thr kinase. PID and protein phosphatase 2A (PP2A) modulate antagonistically the localization of PIN proteins through phosphorylation of PIN proteins.17 These results indicate that the translocation of PIN proteins modulated by PID and PP2A are involved in gravitropic signal transduction. Although changes in the gravity vector should be transduced into the activation of PID and PP2A in gravity perception and/or signal transduction, the underlying molecular mechanisms are still unclear.
Earlier studies demonstrated that gravistimulation induces changes in cytoplasmic pH,18,19 inositol 1,4,5-trisphosphate (InsP3)20 and calcium concentration ([Ca2+]c)21 in Arabidopsis, suggesting that these signaling molecules are involved in the early phase of gravitropic responses. Recently, [Ca2+]c-increases induced by gravistimulation were investigated in more detail in Arabidopsis seedlings expressing the Ca2+-sensitive luminescent protein, apoaequorin.22,23 Gravistimulation by turning plants 180° induced a biphasic [Ca2+]c-increase lasting for several minutes.21,23 The kinetic analyses of the [Ca2+]c-increase revealed that the initial and following slow [Ca2+]c-increases are specific for rotational motion of the plants and changes in the gravity vector, respectively.23 The second [Ca2+]c-increase was observed only in hypocotyls and petioles,23 which are spatially related to the shoot gravitropic organs.1,24 Furthermore, increases in the gravitational acceleration by centrifugation caused a monophasic [Ca2+]c-increase lasting for several minutes in Arabidopsis seedlings, of which time-course closely resembles the second [Ca2+]c-increase by 180°-gravistimulation.22 The presentation time, a minimum time of gravistimulation to elicit gravitropism, is estimated at less than several minutes in a variety of species,25 indicating that gravity perception is carried out in this period. Collectively, the second [Ca2+]c-increase may also be eligible to be a potential candidate for the signaling event of gravity perception in shoots of Arabidopsis seedlings.
Although these two factors, auxin and [Ca2+]c, seem to be critical for gravity perception,26,27 their relationship is poorly understood. We consider this issue here mainly based on our recent results regarding the effects of auxin-transport and vesicle-trafficking inhibitors on the [Ca2+]c-increase induced by gravistimulation.
Gravi-Induced Auxin Transport and [Ca2+]c-Increase
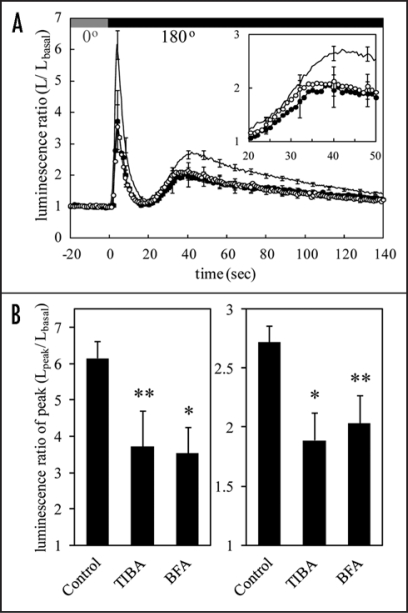
An earlier work showed that the biphasic [Ca2+]c-increase induced by 135°-gravistimulation is strongly suppressed by the conventional auxin transport inhibitors, naphthylphthalamic acid (NPA) and 2,3,5-triiodobenzoic acid (TIBA), at the concentration of 100 µM.21 We confirmed the inhibitory effect of TIBA on the [Ca2+]c-increase at a lower concentration (10 µM) in our setup,23 since the specificity of these inhibitors is uncertain at higher concentrations. Furthermore, effects of the fungal toxin brefeldin A (BFA), a vesicle trafficking inhibitor, on the [Ca2+]c-increase were examined to clarify the relationship between the [Ca2+]c-increase and membrane trafficking. Arabidopsis thaliana seedlings expressing the Ca2+-sensitive luminescent protein, apoaequorin, were mounted under a photomultiplier tube in a light-tight dark box and were subjected to gravistimulation. Peak amplitudes of the initial and second [Ca2+]c-increases induced by 180°-gravistimulation were attenuated by extracellularly applied TIBA and BFA, whereas the rising phase of the second [Ca2+]c-increase including its onset and rate of rise was not significantly affected (Fig. 1A and B). It looks as if the second [Ca2+]c-increase is suddenly suppressed on the way to its peak by something caused by the inhibitors. The gravi-induced asymmetrical distribution of auxin is inhibited by NPA or TIBA in Tobacco stems,4 Arabidopsis hypocotyls and roots6,28,29 as well as by BFA in Arabidopsis roots.7 Exogenously applied auxin (e.g., IAA and 2,4-D) causes a [Ca2+]c-increase in maize coleoptiles, parsley hypocotyls30 and Arabidopsis seedlings.21 Therefore, it seems plausible that the asymmetrically distributed auxin causes the second [Ca2+]c-increase during gravistimulation, as discussed previously.21 However, our kinetic analysis below proposes an alternative interpretation of these observations.
Figure 1.
The effects of auxin-transport and vesicle-trafficking inhibitors on the initial and second [Ca2+]c-increases. (A) The auxin transport inhibitor (TIBA) and vesicle trafficking inhibitor (BFA) were extracellularly applied to Arabidopsis seedlings for 2 h at the concentration of 10 µM. Each averaged trace shows changes in luminescence ratio induced by gravistimulation at the speed of 6 rpm in control (solid line; n = 39), TIBA- (closed circle; n = 16) and BFA-treated seedlings (open circle; n = 17). Inset shows an enlargement of the second [Ca2+]c-increase. (B) The peak amplitudes of the initial (left) and second (right) [Ca2+]c-increases are shown. Data represent means ± SEs, **p < 0.05; *p < 0.01, the two-tailed Student's t-test between control and each TIBA and BFA-treated seedlings.
The inhibitory effects of TIBA and BFA on the auxin redistribution are due to an inhibition of trafficking of membrane proteins such as PIN proteins between the plasma membrane and endosomes.6,28,29,31 The polar auxin transport will be reduced by inhibition of the PIN protein translocation during gravistimulation.6 If [Ca2+]c increases in response to the polar auxin transport, TIBA and BFA must inhibit the rising phase of the second [Ca2+]c-increase through the reduction of the polar auxin flux, resulting in a delay in its onset and a decrease in its rate of rise. However, these inhibitors did not affect significantly the rising phase of the second [Ca2+]c-increase at least until a certain time (ca. 35 seconds after gravistimulation) as mentioned above (Fig. 1A inset and Table 1). Furthermore, timecourse of a [Ca2+]c-increase induced by the exogenous auxin is quite different from that of the gravi-induced second [Ca2+]c-increase; [Ca2+]c peaks at around 10 minutes after application of auxin and the [Ca2+]c-increase lasts as long as the applied auxin is present in Arabidopsis seedlings.21 These results suggest that redistribution of auxin is not involved in the early phase of the [Ca2+]c-increase. TIBA and BFA also inhibited the first [Ca2+]c-increase that appears to be independent of changes in the gravity vector,23 suggesting that these inhibitors affect directly/indirectly the molecules responsible for the [Ca2+]c-increase, such as Ca2+ channels rather than for the auxin-related gravitropic responses. Indeed, TIBA greatly reduces ionic currents in maize roots,32 and BFA abolishes a [Ca2+]c-gradient and -oscillation in pollen tubes, indicating that these inhibitors have multiple side effects as previously pointed out.21
Table 1.
The effects of auxin-transport and vesicle-trafficking inhibitors on the onset and rate of rise of the second [Ca2+]c-increase
| onset | rate of rise | ||
| control | (n = 39) | 22.4 ± 0.8 | 0.08 ± 0.01 |
| TIBA | (n = 16) | 23.8 ± 1.3 | 0.06 ± 0.02 |
| BFA | (n = 17) | 21.1 ± 1.3 | 0.07 ± 0.02 |
The onset (sec) was determined as the time from the start of rotation (0 sec) to the minimum signal amplitude between the initial and second [Ca2+]c peaks. The rate of rise was estimated as a slope of the second [Ca2+]c-increase between 20 to 35 sec by linear regression. Data represent means ± SEs. There is no statistical difference between control and each TIBA and BFA-treated seedlings.
Polar auxin transport was detected in tomato hypocotyls5 between 5 and 10 minutes after gravistimulation as well as in maize coleoptiles between 30 and 40 minutes.33 Our gravi-induced second [Ca2+]c-increase peaked at around 40 seconds from the start of rotation and declined to the basal level in approximately 5 minutes (Fig. 1A), which is much shorter than the time to detect changes in the polar auxin transport. Calcium chelators such as ethylenediaminetetraacetic acid (EDTA) or ethylene glycol bis(beta-aminoethyl ether) -N,N,N',N'-tetraacetic acid (EGTA) inhibit basipetal auxin transport in sunflower stems34 and maize roots.35 Gravi-induced polar auxin transport and gravitropism in maize roots are almost completely inhibited by EGTA.36 These pharmacological results suggest that calcium ion plays an important role in the polar auxin transport.
PID, a modulator for localization of PIN proteins, interacts with two calcium binding proteins, the calmodulin-related protein TOUCH3 (TCH3) and PID-BINDING PROTEIN 1 (PBP1) that contains putative EF-hand motifs, in a calcium-dependent manner.37 Autophosphorylation of PID is enhanced in the presence of PBP1, whereas both TCH3 and PBP1 are not directly phosphorylated by PID, supporting the possibility that the calcium-binding proteins are upstream signaling molecules to regulate the PID kinase activity in Arabidopsis. Overexpression of PID induces an abnormal localization of PIN proteins and collapsed the root meristem by loss of auxin gradients,16,17 which was enhanced by the potential inhibitors of mechanosensitive calcium permeable channels, Gd3+ and La3+, and calmodulin inhibitor, tetracain.37 These results imply that [Ca2+]c negatively regulates the PID activity together with the calcium-binding proteins and affects the localization of PIN proteins in Arabidopsis. In fact, PID activity is directly inhibited by Ca2+ in vitro.38 If the gravi-induced second [Ca2+]c-increase is an upstream signal of the polar auxin transport, it may facilitate the translocation of PIN proteins through the calcium-binding proteins. PIN3 is translocated at the lower side of the plasma membrane in the columella cells of Arabidopsis roots within several minutes after gravistimulation,6 which is roughly consistent with the time course of the gravi-induced [Ca2+]c-increase (Fig. 1A), supporting the above idea that [Ca2+]c-increase is an upstream event of auxin transport. Further studies are required to clarify the time-course of the PIN protein translocation and the following asymmetrical auxin distribution during gravistimulation.
Conclusion
It is generally accepted that calcium ion plays a critical role in plant organ gravitropism.27 [Ca2+]c-increases in response to gravistimulation have been reported in a variety of plants.21,23,39 However, the role of [Ca2+]c-increases in gravitropism and its relationship with auxin have remained largely obscure. We show here that the [Ca2+]c-increases are possibly involved in an auxin-related gravitropic responses. Based on our kinetic analysis, it is likely that the second [Ca2+]c-increase is involved in asymmetrical localization of PIN proteins during gravistimulation. Moreover, the [Ca2+]c-increase and Ca2+-dependent regulation of PID can be located upstream of the gravi-induced asymmetrical distribution of auxin. However, as discussed above, we do not exclude completely the possibility that the polar auxin transport causes a [Ca2+]c-increase. The late component of the second [Ca2+]c-increase (>ca. 35 seconds after gravistimulation) may be a downstream of the asymmetrical auxin distribution, since its peak amplitude is attenuated by the inhibition of auxin transport. It seems reasonable that Ca2+ interacts reciprocally with auxin at certain stages of gravitropic response in Arabidopsis. Exploring the translocation of PIN proteins with high time resolution is essential for understanding not only the relationship between the [Ca2+]c-increase and auxin but also the molecular mechanisms underlying the gravity perception in plants.
Acknowledgements
We thank Dr. M. Kuse and Prof. M. Isobe (Nagoya University, Japan) for synthesizing coelenterazine, Prof. A. Trewavas (University of Edinburgh, UK) for providing us seeds of transgenic Arabidopsis thaliana Col-0 expressing apoaequorin. This work was supported in part by Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists (to M.T.), ICORP/SORST (Japan Science and Technology Agency, to M.S.), Grants-in-aid for General Scientific Research (#14580769 to H.T. and #13480216 to M.S.), Scientific Research on Priority Areas (#15086270 to M.S.) and Creative Research (#16GS0308 to M.S.) from the Ministry of Education Science Sports and Culture and a grant from Japan Space Forum (to H.T. and M.S.).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6339
References
- 1.Blancaflor EB, Masson PH. Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol. 2003;133:1677–1690. doi: 10.1104/pp.103.032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darwin C. The power of movement in plants. London: John Murray; 1880. [Google Scholar]
- 3.Went FW, Thimann KV. Phytohormones. New York: MacMillan; 1937. [Google Scholar]
- 4.Li Y, Hagen G, Guilfoyle TJ. An auxin-responsive promoter is differentially induced by auxin gradients during tropisms. Plant Cell. 1991;3:1167–1175. doi: 10.1105/tpc.3.11.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison MA, Pickard BG. Auxin asymmetry during gravitropism by tomato hypocotyls. Plant Physiol. 1989;89:652–657. doi: 10.1104/pp.89.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 7.Ottenschlager I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA. 2003;100:2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swarup R, Kramer EM, Perry P, Knox K, Leyser HM, Haseloff J, Beemster GT, Bhalerao R, Bennett MJ. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol. 2005;7:1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- 9.Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 10.Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 12.Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 13.Harrison BR, Masson PH. ARL2, ARG1 and PIN3 define a gravity signal transduction pathway in root statocytes. Plant J. 2008;53:380–392. doi: 10.1111/j.1365-313X.2007.03351.x. [DOI] [PubMed] [Google Scholar]
- 14.Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Galweiler L, Palme K, Jurgens G. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;286:316–318. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- 15.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 16.Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, Hooykaas PJ, Palme K, Offringa R. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- 17.Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, Schwab R, Weigel D, Meyerowitz EM, Luschnig C, Offringa R, Friml J. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–1056. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Scott AC, Allen NS. Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol. 1999;121:1291–1298. doi: 10.1104/pp.121.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell. 2001;13:907–921. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perera IY, Hung CY, Brady S, Muday GK, Boss WF. A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol. 2006;140:746–760. doi: 10.1104/pp.105.075119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plieth C, Trewavas AJ. Reorientation of seedlings in the earth's gravitational field induces cytosolic calcium transients. Plant Physiol. 2002;129:786–796. doi: 10.1104/pp.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Hypergravity stimulation induces changes in intracellular calcium concentration in Arabidopsis seedlings. Adv Space Res. 2007;39:1190–1197. [Google Scholar]
- 23.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 2008;146:505–514. doi: 10.1104/pp.107.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mano E, Horiguchi G, Tsukaya H. Gravitropism in leaves of Arabidopsis thaliana (L.) Heynh. Plant Cell Physiol. 2006;47:217–223. doi: 10.1093/pcp/pci237. [DOI] [PubMed] [Google Scholar]
- 25.Perbal G, Jeune B, Lefranc A, Carnero-Diaz E, Driss-Ecole D. The dose-response curve of the gravitropic reaction: a re-analysis. Physiol Plant. 2002;114:336–342. doi: 10.1034/j.1399-3054.2002.1140302.x. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinclair W, Trewavas AJ. Calcium in gravitropism. A re-examination. Planta. 1997;203:85–90. doi: 10.1007/pl00008120. [DOI] [PubMed] [Google Scholar]
- 28.Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jurgens G, Geldner N, Friml J. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 29.Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, Paciorek T, Petrasek J, Seifertova D, Tejos R, Meisel LA, Zazimalova E, Gadella TW, Jr, Stierhof YD, Ueda T, Oiwa K, Akhmanova A, Brock R, Spang A, Friml J. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA. 2008;105:4489–4494. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehring CA, Irving HR, Parish RW. Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci USA. 1990;87:9645–9649. doi: 10.1073/pnas.87.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 32.Collings DA, White RG, Overall RL. Ionic current changes associated with the gravity-induced bending response in roots of Zea mays L. Plant Physiol. 1992;100:1417–1426. doi: 10.1104/pp.100.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker KE, Briggs WR. Transport of indole-3-acetic acid during gravitropism in intact maize coleoptiles. Plant Physiol. 1990;94:1763–1769. doi: 10.1104/pp.94.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dela Fuente RK, Leopold AC. A role for calcium in auxin transport. Plant Physiol. 1973;51:845–847. doi: 10.1104/pp.51.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasenstein KH, Evans ML. Effects of cations on hormone transport in primary roots of Zea mays. Plant Physiol. 1988;86:890–894. doi: 10.1104/pp.86.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young LM, Evans ML. Calcium-dependent asymmetric movement of 3H-indole-3-acetic acid across gravistimulated isolated root caps of maize. Plant Growth Regul. 1994;14:235–242. [Google Scholar]
- 37.Benjamins R, Ampudia CS, Hooykaas PJ, Offringa R. PINOID-mediated signaling involves calcium-binding proteins. Plant Physiol. 2003;132:1623–1630. doi: 10.1104/pp.103.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zegzouti H, Anthony RG, Jahchan N, Bogre L, Christensen SK. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:6404–6409. doi: 10.1073/pnas.0510283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gehring CA, Williams DA, Cody SH, Parish RW. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature. 1990;345:528–530. doi: 10.1038/345528a0. [DOI] [PubMed] [Google Scholar]