Abstract
In our recent paper in Journal of Experimental Botany, we examined the effects of cpr5/old1 mutations and CPR5 overexpression on Arabidopsis growth and development.1 We found that CPR5 is important for early plant growth but promotes senescence at late development and hence proposed it as a senescence-regulatory gene as predicted by the Evolutionary Theory of Senescence derived from studies on animal ageing. One of the key unsolved issues is how CPR5 contributes to the early plant growth and development. Here we discuss the possible cellular functions of CPR5.
Key words: CPR5, cell death, senescence, reactive oxygen species (ROS)
The Pleiotropic Phenotypes of cpr5 Mutations
CPR5 stands for CONSTITUTIVE EXPRESSER OF PR GENES5. The gene was initially identified in screens for mutants with enhanced disease resistance.2,3 Several more cpr5 alleles were subsequently identified in screens for mutants with altered trichome development,4 dark induced senescence (hys1 alleles)5 and ethylene-induced senescence (old1 alleles).1,6 So far, described alterations in cpr5 mutants include (1) enhanced constitutive expression of PR genes, (2) elevated salicylic acid and jasmonic acid levels, (3) hypersensitivities to sugar, ABA, ethylene and jasmonic acid, (4) accelerated leaf senescence, (5) spontaneous lesion mimic cell death, (6) defective cell proliferation, endoreduplication and trichome development, (7) reduced plant growth and reproduction fecundity.1–7 Thus, cpr5 mutations possess pleiotropic phenotypes indicating that CPR5 is an essential-for-life gene in Arabidopsis.
How is CPR5 related to all of these processes? Several double mutants were constructed to dissect how the altered phenotypes in cpr5 mutants are related to the known signalling pathways such as salicylic acid, ethylene, jasmonic acid, sugar and ABA (Jing H-C, Dijkwel PP, unpublished data)8,9 Blocking glucose sensing by knocking out HXK1 (hexokinase1) could partially alleviate the glucose hypersensitive phenotype of the cpr5 mutant, but did not affect the early onset of senescence and defect in trichome development.9 This is consistent to our results showing that blocking a particular signalling pathway did not affect the alterations in other signalling pathways in cpr5 mutants.11 For instance, cpr5-induced ethylene hypersensitivity was converted into ethylene insensitivity in cpr5ein2 double mutants, but cpr5ein2 seedlings still exhibited hypersensitivity to sugar, ABA and jasmonic acid. Similarly, cpr5abi4 double mutants were sugar insensitive but were still hypersensitive to ethylene and jasmonic acid. Furthermore, all examined double mutants so far had no effects on cpr5-induced lesion mimic cell death and premature senescence as well as reduced cell proliferation and trichome development. It appears that CPR5 independently controls multiple cellular processes.
Cellular Functions of CPR5: A Regulator of ROS Gene Network
There are at least two possible cellular mechanisms to explain the observed pleiotropy in cpr5 mutants. CPR5 can be a master regulator of a general signal transduction pathway that affects many processes. Alternatively, CPR5 can independently interact with many signalling molecules. Defining the earliest alterations in cellular events in pre-symptom cpr5 mutants may help understand the cellular functions of CPR5. We therefore examined the transcriptomic and proteomic profiles of pre-symptom cpr5 mutants.10,11 Using 5-fold increase as a cut-off threshold three of the five universal ROS marker genes, 16 of the 27 genes induced by six ROS treatments and one third of the ROS-dependent putative transcription factors were upregulated in cpr5 mutants. Proteins clearly exhibiting increased abundance were predominantly members of the detoxifying enzyme family of glutathione S-transferases (GSTs). Thus, pre-symptom cpr5 mutants are under high cellular oxidative stress. Consistent with this, the presence of reactive oxygen species was recorded in cpr5 mutants using nitro blue tetrazolium staining.2
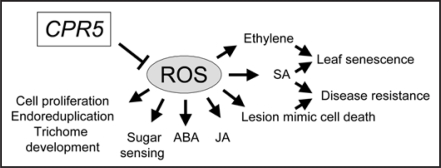
These observations may link the various pleiotropic phenotypes in cpr5 mutants to the alteration of cellular ROS production, scavenging and redox balance. ROS are by-products of essential cellular metabolic processes.12 Recent evidence shows that ROS are a part of master signalling transduction pathways involved in the regulation of many growth and development as well as biotic and abiotic stress responses in plants.13 ROS have also been implicated in regulating responses to plant hormones such as auxin, ethylene, gibberellic acids, and ABA as well as cell death.14,15 We hence propose that CPR5 is a master regulator of cellular ROS status and/or signalling, which has close and complex interactions with other signalling networks to control cell proliferation, endoreduplication and trichome development, responses to ethylene, sugar, jasmonic acid and ABA, cell death and senescence as well as disease resistance (Fig. 1).
Figure 1.
A diagram showing CPR5 as a master regulator of cellular ROS status and signalling.
CPR5 Mode of Action: An Open Question
Arabidopsis CPR5 encodes a protein containing an amino terminus bipartite nuclear localisation signal and five transmembrane domains at the carboxy terminus. Blast search of currently available genome and protein databases identified putative CPR5 homologues in higher plants as well as in mosses (Physcomitrella patens) which separated from plants 400 million years ago. Thus, CPR5 is an ancient, plant unique gene.
The CPR5 transcript is constitutively expressed throughout the plant and increases levels at late development.1,4,5 The amino acid sequence of CPR5 does not resemble any proteins of known function but its structure resembles several proteins in animals and bacteria.16–20 Cellular localisation of CPR5 needs to be solved in order to accurately dissect how it acts in a plant cell. It is also necessary to identify the immediate interaction partners of CPR5 and domain swapping and chopping experiments should provide clues on the functional domains of CPR5. Knock-down/overexpression of CPR5 in a developmental manner using inducible promoters may show how CPR5 changes functions during development.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5708
References
- 1.Jing HC, Anderson L, Sturre MJ, Hille J, Dijkwel PP. Arabidopsis CPR5 is a senescence-regulatory gene with pleiotropic functions as predicted by the evolutionary theory of senescence. J Exp Bot. 2007;58:3885–3894. doi: 10.1093/jxb/erm237. [DOI] [PubMed] [Google Scholar]
- 2.Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boch J, Verbsky ML, Robertson TL, Larkin JC, Kunkel BN. Analysis of resistance gene-mediated defense responses in Arabidopsis thaliana plants carrying a mutation in CPR5. MPMI. 1998;11:1196–1206. [Google Scholar]
- 4.Kirik V, Bouyer D, Schobinger U, Bechtold N, Herzog M, Bonneville JM, Hulskamp M. CPR5 is involved in cell proliferation and cell death control and encodes a novel transmembrane protein. Curr Biol. 2001;11:1891–1895. doi: 10.1016/s0960-9822(01)00590-5. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida S, Ito M, Nishida I, Watanabe A. Identification of a novel gene HYS1/CPR5 that has a repressive role in the induction of leaf senescence and pathogen-defence responses in Arabidopsis thaliana. Plant J. 2002;29:427–437. doi: 10.1046/j.0960-7412.2001.01228.x. [DOI] [PubMed] [Google Scholar]
- 6.Jing HC, Sturre MJ, Hille J, Dijkwel PP. Arabidopsis onset of leaf death mutants identify a regulatory pathway controlling leaf senescence. Plant J. 2002;32:51–63. doi: 10.1046/j.1365-313x.2002.01400.x. [DOI] [PubMed] [Google Scholar]
- 7.Heidel AJ, Clarke JD, Antonovics J, Dong X. Fitness costs of mutations affecting the systemic acquired resistance pathway in Arabidopsis thaliana. Genetics. 2004;168:2197–2206. doi: 10.1534/genetics.104.032193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell. 2000;12:2175–2190. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aki T, Konishi M, Kikuchi T, Fujimori T, Yoneyama T, Yanagisawa S. Distinct modulations of the hexokinase1-mediated glucose response and hexokinase1-independent processes by HYS1/CPR5 in Arabidopsis. J Exp Bot. 2007;58:3239–3248. doi: 10.1093/jxb/erm169. [DOI] [PubMed] [Google Scholar]
- 10.Hebeler R, Oeljeklaus S, Reidegeld KA, Eisenacher M, Stephan C, Sitek B, Stuhler K, Meyer HE, Sturre MJ, Dijkwel PP, et al. Study of early leaf senescence in Arabidopsis thaliana by quantitative proteomics using reciprocal 14N/15N-labeling and difference gel electrophoresis. Mol Cell Proteomics. 2008;7:108–120. doi: 10.1074/mcp.M700340-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Jing HC, Hebeler R, Oeljeklaus S, Sitek B, Stühler K, Meyer HE, Sturre MJG, Hille J, Warscheid B, Dijkwel PP. Early leaf senescence is associated with an altered cellular redox balance in Arabidopsis cpr5/old1 mutants. Plant Biol. 2008 doi: 10.1111/j.1438-8677.2008.00087.x. In press. [DOI] [PubMed] [Google Scholar]
- 12.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 13.Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antioxid Redox Signal. 2006;8:1757–1764. doi: 10.1089/ars.2006.8.1757. [DOI] [PubMed] [Google Scholar]
- 14.Kwak JM, Nguyen V, Schroeder JI. The role of reactive oxygen species in hormonal responses. Plant Physiol. 2006;141:323–329. doi: 10.1104/pp.106.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urban S, Freeman M. Intramembrane proteolysis controls diverse signalling pathways throughout evolution. Curr Opin Genet Dev. 2002;12:512–518. doi: 10.1016/s0959-437x(02)00334-9. [DOI] [PubMed] [Google Scholar]
- 17.Urban S, Schlieper D, Freeman M. Conservation of intramembrane proteolytic activity and substrate specificity in prokaryotic and eukaryotic rhomboids. Curr Biol. 2002;12:1507–1512. doi: 10.1016/s0960-9822(02)01092-8. [DOI] [PubMed] [Google Scholar]
- 18.Burke B, Shanahan C, Salina D, Crisp M. Aspects of nuclear envelope dynamics in mitotic cells. Novartis Found Symp. 2005;264:22–30. discussion 30–4, 227–30. [PubMed] [Google Scholar]
- 19.Enarson P, Rattner JB, Ou Y, Miyachi K, Horigome T, Fritzler MJ. Autoantigens of the nuclear pore complex. J Mol Med. 2004;82:423–433. doi: 10.1007/s00109-004-0554-z. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison CJ, Alvarez-Reyes M, Vaughan OA. Lamins in disease: why do ubiquitously expressed nuclear envelope proteins give rise to tissue-specific disease phenotypes? J Cell Sci. 2001;114:9–19. doi: 10.1242/jcs.114.1.9. [DOI] [PubMed] [Google Scholar]