Abstract
Low temperature negatively affects plant growth and metabolism. Plant responses to cold involve massive transcriptional changes, and much effort has been made to identify these changes and their contribution to freezing tolerance. However, the influence of differences in environmental and developmental factors between experiments had not been investigated. We found that diurnal- and circadian-regulated genes are responsible for the majority of variation between experiments. Moreover, we demonstrated that the cyclic expression pattern of circadian clock components is affected by cold and that the cold induction of many transcription factors is dependent on the time of day. This means that genes identified so far as cold responsive are dependent on the time of day the experiment was performed and that paired diurnal controls are not sufficient to correct for this effect. Ongoing work to dissect the biological relevance of cold-diurnal regulatory interactions demonstrated that some circadian mutants have altered freezing tolerance but that time-of-day appears not to affect freezing tolerance.
Key words: cold stress, circadian clock, transcription factors expression, gating, cold-diurnal regulation
Influence of Circadian and Diurnal Regulation on the Identification of Cold-Responsive Genes
Extending our previous analyses of cold responsive transcripts,1,2 we used microarray data from public sources as well as from our own experiments and identified massive differences in cold-responsive genes between independent studies. Diurnally regulated genes were the dominant source of these variations, highlighting that measures taken to minimize or eliminate the effect of diurnal or circadian regulation are insufficient.
To gain broader insights into the molecular basis of the interaction between cold and the circadian clock, we analyzed the expression of clock components and clock output genes during cold treatments (Fig. 1). The majority of oscillator components, after an initial cold response, showed diurnal cycles with dramatically reduced amplitude but similar peak expression in cold as under control conditions. Under circadian conditions in the cold, cycles stopped. Paired controls are insufficient to avoid circadian or diurnal variations because genes in control samples show normal amplitude cycles, thus diurnal and circadian regulated genes will clearly exhibit differences in relative changes in gene expression. Interestingly, LUX expression was maintained at the same amplitude under normal and cold conditions pointing to a unique regulation among the measured components. The probable importance of LUX is supported by the atypical arrhythmic phenotype of the single mutant.3,4
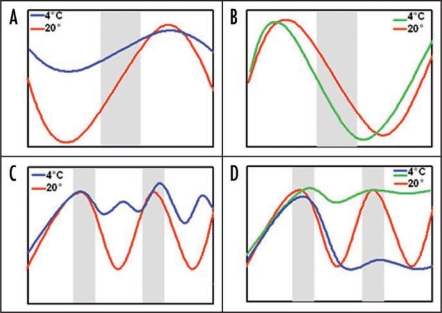
Figure 1.
Summary of the effect of cold in the expression of circadian clock genes. In diurnal conditions (A and B) cold dampens the cycle amplitude of clock components, such as lhy, cca1 (A, blue line). However, cold has an unique effect in lux expression (B, green line). In circadian conditions (C and D), the expression of clock components becomes arrhythmic (C, blue line). Cold generates different effects on the expression of output marker genes. They can be clamped to their maximum circadian expression level, as was observed for CAB2 (D, green line) or to their minimum, such as CAT3 (D, blue line). Grey bars represent night (A and B) or subjective night (C and D).
Temperature compensation of circadian clocks allows the maintenance of robust rhythms over a broad range of physiological temperatures. It has been shown that a balance between CCA1 and GI has a relevant role on this mechanism at lower temperatures down to 12°C.5 In our cold treatment, GI diurnal cycles decrease their amplitude almost completely, and in circadian conditions GI expression increases and stops to cycle. Since GI was implicated in both constitutive freezing tolerance and cold acclimation in Arabidopsis, via a CBF-independent pathway6 it is possible that transcriptional changes of GI, and other oscillator components, might be of relevance for the cold response and freezing tolerance.
Time of Day Dependence of Cold Response
As a consequence of the circadian control of gene expression, a stimulus of equal strength applied at different times of the day can result in a different magnitude of the response. This effect is known as “gating”. The gated cold induction of low temperature-induced transcription factors, such as CBF1-3, RAV1 and ZAT12 was previously demonstrated.7 Interestingly, we demonstrated widespread diurnal gating of cold responsive transcription factors, with more and stronger induction, to higher absolute transcript abundance, in the morning (2 hours after dawn) than evening (14 hours after dawn). Comparison of initial transcript levels in the morning and evening revealed that this is due to an increased cold-induction, since the transcript levels for most gated genes under control conditions were higher in the evening. Interesting, although some transcription factor families, such as AP2/EREBP and C2C2(Zn) CO-like were particularly prominent, our data show that circadian gating is a general phenomenon and thus require a general regulatory mechanism, for example diurnal chromatin changes.8
Concluding Remarks
The biological meaning of changes in cyclic expression of clock components or output genes is intriguing. If circadian oscillations are stopped by cold and genes are maintained at different expression levels, they could make particular contributions to the cold response. The contribution of the circadian clock to plant fitness under normal growth conditions has been established.9,10 However, the role of the circadian clock for plants at low temperature is unknown. We are currently resolving this using measurements of growth, competitive advantage and freezing tolerance of clock mutants and wild-type plants. In this regard, we have observed that the cca1 single and cca1/lhy double mutants are more sensitive to freezing in comparison to wild-type plants. However, the function of the massive gating in cold induction is less clear. The clock might affect the sensitivity of the regulation of CBF1-3, as was previously suggested.7 It may be that plants have particular requirements to face low temperature at different times of the day. To investigate whether such differences in the gating of the transcriptional response have an effect on freezing tolerance, we determined the ability of plants to cold acclimate after transfer in the morning or evening. Twenty-four hours of cold acclimation assured the same duration of light, and as the plants do not fully acclimate by this time, temporal differences could be revealed. We quantified freezing tolerance by measuring leaf electrolyte leakage11 before and after cold acclimation. Although significant acclimation occurred, differences in the nonacclimated (LT50: −6.85°C in the morning; −6.21°C in the evening), or acclimated (LT50: −8.29°C in the morning; −7.82°C in the evening) tolerance at the different times was not significant (unpublished data). Obviously, the physiological significance of diurnal gating of the cold induction of transcription factors requires further investigation. Currently, we are characterizing the system-wide metabolic and transcriptional changes throughout the day at low temperature and their significance in configuring plant responses to low temperature.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/6340
References
- 1.Hannah MA, Wiese D, Freund S, Fiehn O, Heyer AG, Hincha DK. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol. 2006;142:98–112. doi: 10.1104/pp.106.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannah MA, Heyer AG, Hincha DK. A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet. 2005;1:26. doi: 10.1371/journal.pgen.0010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onai K, Ishiura M. PHYTOCLOCK 1 encoding a novel GARP protein essential for the Arabidopsis circadian clock. Genes Cells. 2005;10:963–972. doi: 10.1111/j.1365-2443.2005.00892.x. [DOI] [PubMed] [Google Scholar]
- 4.Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc Natl Acad Sci USA. 2005;102:10387–10392. doi: 10.1073/pnas.0503029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould PD, Locke JC, Larue C, Southern MM, Davis SJ, et al. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell. 2006;18:1177–1187. doi: 10.1105/tpc.105.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao S, Ye M, Jiang S. Involvement of GIGANTEA gene in the regulation of the cold stress response in Arabidopsis. Plant Cell Rep. 2005;24:683–690. doi: 10.1007/s00299-005-0061-x. [DOI] [PubMed] [Google Scholar]
- 7.Fowler SG, Cook D, Thomashow MF. Low temperature induction of Arabidopsis CBF1, 2 and 3 is gated by the circadian clock. Plant Physiol. 2005;137:961–968. doi: 10.1104/pp.104.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perales M, Mas P. A functional link between rhythmic changes in chromatin structure and the Arabidopsis biological clock. Plant Cell. 2007;19:2111–2123. doi: 10.1105/tpc.107.050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green RM, Tingay S, Wang ZY, Tobin EM. Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol. 2002;129:576–584. doi: 10.1104/pp.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd AN, Salathia N, Hall A, Kevei E, Toth R, et al. Plant circadian clocks increase photosynthesis, growth, survival and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 11.Rohde P, Hincha DK, Heyer AG. Heterosis in the freezing tolerance of crosses between two Arabidopsis thaliana accessions (Columbia-0 and C24) that show differences in non-acclimated and acclimated freezing tolerance. Plant J. 2004;38:790–799. doi: 10.1111/j.1365-313X.2004.02080.x. [DOI] [PubMed] [Google Scholar]